Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies
- PMID: 33815418
- PMCID: PMC8012774
- DOI: 10.3389/fimmu.2021.655697
Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies
Abstract
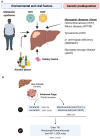
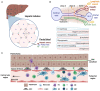
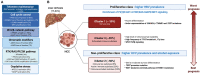
Hepatocellular carcinoma (HCC) is the most common liver tumor and among the deadliest cancers worldwide. Advanced HCC overall survival is meager and has not improved over the last decade despite approval of several tyrosine kinase inhibitors (TKi) for first and second-line treatments. The recent approval of immune checkpoint inhibitors (ICI) has revolutionized HCC palliative care. Unfortunately, the majority of HCC patients fail to respond to these therapies. Here, we elaborate on the immune landscapes of the normal and cirrhotic livers and of the unique HCC tumor microenvironment. We describe the molecular and immunological classifications of HCC, discuss the role of specific immune cell subsets in this cancer, with a focus on myeloid cells and pathways in anti-tumor immunity, tumor promotion and immune evasion. We also describe the challenges and opportunities of immunotherapies in HCC and discuss new avenues based on harnessing the anti-tumor activity of myeloid, NK and γδ T cells, vaccines, chimeric antigen receptors (CAR)-T or -NK cells, oncolytic viruses, and combination therapies.
Keywords: NASH; cirrhosis; immune checkpoint inhibitors; immunosuppression; immunotherapy; inflammation; tumor microenvironment; tumor-associated macrophages.
Copyright © 2021 Giraud, Chalopin, Blanc and Saleh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma.J Exp Clin Cancer Res. 2019 Sep 9;38(1):396. doi: 10.1186/s13046-019-1396-4. J Exp Clin Cancer Res. 2019. PMID: 31500650 Free PMC article. Review.
-
Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma.Front Immunol. 2021 Nov 26;12:783236. doi: 10.3389/fimmu.2021.783236. eCollection 2021. Front Immunol. 2021. PMID: 34899747 Free PMC article. Review.
-
Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: current status and prospectives.J Hematol Oncol. 2024 Apr 29;17(1):25. doi: 10.1186/s13045-024-01549-2. J Hematol Oncol. 2024. PMID: 38679698 Free PMC article. Review.
-
Immunodiagnostic Biomarkers for Hepatocellular Carcinoma (HCC): The First Step in Detection and Treatment.Int J Mol Sci. 2021 Jun 7;22(11):6139. doi: 10.3390/ijms22116139. Int J Mol Sci. 2021. PMID: 34200243 Free PMC article. Review.
-
In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma.Front Immunol. 2021 May 7;12:650486. doi: 10.3389/fimmu.2021.650486. eCollection 2021. Front Immunol. 2021. PMID: 34025657 Free PMC article. Review.
Cited by
-
Analysis of ZNF208 Polymorphisms on the Clinicopathologic Characteristics of Asian Patients with Hepatocellular Carcinoma.J Cancer. 2024 Aug 13;15(16):5183-5190. doi: 10.7150/jca.98520. eCollection 2024. J Cancer. 2024. PMID: 39247597 Free PMC article.
-
Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies.Int J Mol Sci. 2024 Jun 21;25(13):6830. doi: 10.3390/ijms25136830. Int J Mol Sci. 2024. PMID: 38999940 Free PMC article. Review.
-
A novel immune-related gene signature predicts the prognosis of hepatocellular carcinoma.Front Oncol. 2022 Sep 15;12:955192. doi: 10.3389/fonc.2022.955192. eCollection 2022. Front Oncol. 2022. PMID: 36185203 Free PMC article.
-
Recent advances of engineered oncolytic viruses-based combination therapy for liver cancer.J Transl Med. 2024 Jan 2;22(1):3. doi: 10.1186/s12967-023-04817-w. J Transl Med. 2024. PMID: 38167076 Free PMC article. Review.
-
Roles of innate lymphoid cells in metabolic and alcohol-associated liver diseases.JHEP Rep. 2023 Nov 11;6(2):100962. doi: 10.1016/j.jhepr.2023.100962. eCollection 2024 Feb. JHEP Rep. 2023. PMID: 38304237 Free PMC article. Review.
References
-
- Global Burden of Disease Cancer C . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-Adjusted life-Years for 29 cancer groups:1990 to 2017: a Systematic analysis for the global burden of disease study. JAMA Oncol. (2019) 5:1749–68. 10.1001/jamaoncol.2019.2996 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

