Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells
- PMID: 33815531
- PMCID: PMC8008763
- DOI: 10.33073/pjm-2021-009
Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells
Abstract
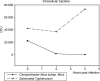
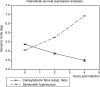
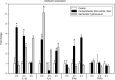
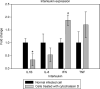
Campylobacter fetus subsp. fetus is the causal agent of sporadic abortion in bovines and infertility that produces economic losses in livestock. In many infectious diseases, the immune response has an important role in limiting the invasion and proliferation of bacterial pathogens. Innate immune sensing of microorganisms is mediated by pattern-recognition receptors (PRRs) that identify pathogen-associated molecular patterns (PAMPs) and induces the secretion of several proinflammatory cytokines, like IL-1β, TNF-α, and IL-8. In this study, the expression of IL-1β, TNF-α, IL-8, and IFN-γ in bovine endometrial epithelial cells infected with C. fetus and Salmonella Typhimurium (a bacterial invasion control) was analyzed. The results showed that expression levels of IL-1β and IL-8 were high at the beginning of the infection and decreased throughout the intracellular period. Unlike in this same assay, the expression levels of IFN-γ increased through time and reached the highest peak at 4 hours post infection. In cells infected with S. Typhimurium, the results showed that IL8 expression levels were highly induced by infection but not IFN-γ. In cells infected with S. Typhimurium or C. fetus subsp. fetus, the results showed that TNF-α expression did not show any change during infection. A cytoskeleton inhibition assay was performed to determine if cytokine expression was modified by C. fetus subsp. fetus intracellular invasion. IL-1β and IL-8 expression were downregulated when an intracellular invasion was avoided. The results obtained in this study suggest that bovine endometrial epithelial cells could recognize C. fetus subsp. fetus resulting in early proinflammatory response.
Keywords: bacterial infection; pathogen-host interaction (MesH); pathogenicity; virulence.
© 2021 Lizeth Guadalupe Campos-Múzquiz et al.
Conflict of interest statement
Conflict of interest The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Figures
Similar articles
-
Campylobacter fetus is Internalized by Bovine Endometrial Epithelial Cells.Pol J Microbiol. 2019;68(2):217-224. doi: 10.33073/pjm-2019-022. Pol J Microbiol. 2019. PMID: 31250592 Free PMC article.
-
Interaction between Campylobacter and intestinal epithelial cells leads to a different proinflammatory response in human and porcine host.Vet Immunol Immunopathol. 2014 Nov 15;162(1-2):14-23. doi: 10.1016/j.vetimm.2014.09.003. Epub 2014 Sep 28. Vet Immunol Immunopathol. 2014. PMID: 25307769
-
Evaluation of intracellular survival of Campylobacter fetus subsp. fetus in bovine endometrial cells by qPCR.Iran J Vet Res. 2021 Spring;22(2):94-99. doi: 10.22099/ijvr.2021.38693.5632. Iran J Vet Res. 2021. PMID: 34306105 Free PMC article.
-
Bovine campylobacteriosis: a review.Can Vet J. 1981 Nov;22(11):327-30. Can Vet J. 1981. PMID: 7039808 Free PMC article. Review.
-
Cytokine responses in campylobacteriosis: Linking pathogenesis to immunity.Cytokine Growth Factor Rev. 2018 Jun;41:75-87. doi: 10.1016/j.cytogfr.2018.03.005. Epub 2018 Mar 10. Cytokine Growth Factor Rev. 2018. PMID: 29550265 Review.
References
-
- Al-Amri AI, Botta GA, Tabbara KS, Ismaeel AY, Al-Mahmeed AE, Qareeballa AY, Bin Dayna KM, Bakhiet MO. Campylobacter jejuni induces diverse kinetics and profiles of cytokine genes in INT-407 cells. Saudi Med J. 2008. Apr;29(4):514–519. - PubMed
-
- Arce RM, Diaz PI, Barros SP, Galloway P, Bobetsis Y, Threadgill D, Offenbacher S. Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J Reprod Immunol. 2010b. Mar;84(2):145–153 10.1016/j.jri.2009.11.003 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
