Burden of major gastrointestinal bleeding among oral anticoagulant-treated non-valvular atrial fibrillation patients
- PMID: 33815568
- PMCID: PMC7989114
- DOI: 10.1177/1756284821997352
Burden of major gastrointestinal bleeding among oral anticoagulant-treated non-valvular atrial fibrillation patients
Abstract
Background: Gastrointestinal (GI) bleeding is the most common type of major bleeding associated with oral anticoagulant (OAC) treatment. Patients with major bleeding are at an increased risk of a stroke if an OAC is not reinitiated.
Methods: Non-valvular atrial fibrillation (NVAF) patients initiating OACs were identified from the Centers for Medicare and Medicaid Services (CMS) Medicare data and four US commercial claims databases. Patients who had a major GI bleeding event (hospitalization with primary diagnosis of GI bleeding) while on an OAC were selected. A control cohort of patients without a major GI bleed during OAC treatment was matched to major GI bleeding patients using propensity scores. Stroke/systemic embolism (SE), major bleeding, and mortality (in the CMS population) were examined using Cox proportional hazards models with robust sandwich estimates.
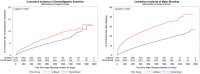
Results: A total of 15,888 patients with major GI bleeding and 833,052 patients without major GI bleeding were included in the study. Within 90 days of the major GI bleed, 58% of patients discontinued the initial OAC treatment. Patients with a major GI bleed had a higher risk of stroke/SE [hazard ratio (HR): 1.57, 95% confidence interval (CI): 1.42-1.74], major bleeding (HR: 2.79, 95% CI: 2.64-2.95), and all-cause mortality (HR: 1.29, 95% CI: 1.23-1.36) than patients without a major GI bleed.
Conclusion: Patients with a major GI bleed on OAC had a high rate of OAC discontinuation and significantly higher risk of stroke/SE, major bleeding, and mortality after hospital discharge than those without. Effective management strategies are needed for patients with risk factors for major GI bleeding.
Keywords: atrial fibrillation; gastrointestinal bleeding; major bleeding; oral anticoagulants; stroke.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: S Deitelzweig is a consultant for Bristol Myers Squibb Company/Pfizer Inc., Daiichi-Sankyo, Portola, and Boehringer Ingelheim, and has been on the speakers’ bureau for Bristol Myers Squibb Company/Pfizer Inc., and Boehringer Ingelheim. A Keshishian is a paid employee of STATinMED Research which is a paid consultant to Pfizer Inc. and Bristol-Myers Squibb Company. A Kang, A Dhamane, N Balachander, L Rosenblatt, and J Jiang are paid employees and shareholders of Bristol-Myers Squibb Company. X Luo and J Mardekian are paid employees and shareholders of Pfizer, Inc. GYH Lip is a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally.
Figures


References
-
- Shea JB, Sears SF. Cardiology patient pages. A patient’s guide to living with atrial fibrillation. Circulation 2008; 117: e340–e343. - PubMed
-
- Colilla S, Crow A, Petkun W, et al.. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol 2013; 112: 1142–1147. - PubMed
-
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. - PubMed
-
- Hansen ML, Sørensen R, Clausen MT, et al.. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170: 1433–1441. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

