Targeting immunoglobulin E in atopic dermatitis: A review of the existing evidence
- PMID: 33815652
- PMCID: PMC8005850
- DOI: 10.1016/j.waojou.2021.100519
Targeting immunoglobulin E in atopic dermatitis: A review of the existing evidence
Abstract
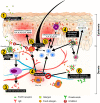
Immunoglobulin E (IgE) plays an essential role in many allergic diseases. This review highlights the role of IgE in atopic dermatitis (AD), a common, chronic, and complex skin inflammation, and the available therapeutic approaches that target IgE in AD. We examine the existing data showing the use of omalizumab, the only biologic anti-IgE therapy available in clinical use, plasma apheresis, and a combination of both therapeutic approaches for the treatment of AD. Existing data on the efficacy of omalizumab in AD are inconclusive. A limited number of randomised controlled studies, few uncontrolled prospective and retrospective reports, as well as multiple case series and case reports observed varying degrees of the efficacy of omalizumab in AD. Omalizumab displays a trend of higher efficacy in AD patients with low IgE levels compared with those with very high-to-extremely high serum IgE concentrations. Plasma apheresis and its combination with omalizumab show good efficacy, even in patients with unusually high serum IgE concentrations. Combining apheresis and anti-IgE treatment may serve as a comprehensive therapeutic approach for patients with elevated levels of IgE. Dedicated clinical studies with robust study designs are needed to establish the therapeutic efficacy of omalizumab in AD.
Keywords: Apheresis; Atopic dermatitis; Biologics; Immunoglobulin E; Omalizumab.
© 2021 The Authors.
Conflict of interest statement
Andreas Wollenberg has received grants, personal fees or nonfinancial support from 10.13039/100006483Abbvie, Almirall, 10.13039/501100010558Beiersdorf, Bioderma, 10.13039/100010795Chugai, Galapagos, 10.13039/501100009754Galderma, Hans Karrer, Leo Pharma, Eli Lilly, L'Oreal, Maruho, 10.13039/501100004628MedImmune, 10.13039/100004336Novartis, 10.13039/100004319Pfizer, 10.13039/100013226Pierre Fabre, 10.13039/100009857Regeneron, 10.13039/501100004286Santen and Sanofi-Aventis. Jean-Philippe Lacour has received grants/research support as an investigator and honoraria, advisory board, or consulting fees from 10.13039/100006483AbbVie, BMS, 10.13039/100008349Boehringer Ingelheim, 10.13039/100006436Celgene, 10.13039/100013988Dermira, 10.13039/501100009754Galderma, Janssen, 10.13039/100004312Eli Lilly and Company, Leo-Pharma, 10.13039/100004334Merck, 10.13039/100004336Novartis, 10.13039/100009857Regeneron, 10.13039/100004337Roche, and 10.13039/100004339Sanofi. Simon Francis Thomsen has been a paid speaker, served on advisory boards and received research support from 10.13039/100006483Abbvie, Almirall, 10.13039/100006436Celgene, Eli Lilly, GSK, Janssen, Leo Pharma, 10.13039/100004336Novartis, 10.13039/100013226Pierre Fabre, 10.13039/100004337Roche, 10.13039/100004339Sanofi and 10.13039/100011110UCB. Xavier Jaumont and Slawomir Lazarewicz are permanent employees of Novartis Pharma AG.
Figures

References
-
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metabol. 2015;66(Suppl 1):8–16. - PubMed
-
- Tsai T.F., Rajagopalan M., Chu C.Y. Burden of atopic dermatitis in Asia. J Dermatol. 2019;46:825–834. - PubMed
-
- Silverberg J.I. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283–289. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

