Inhibition of Inducible Nitric Oxide Synthase (iNOS) by Andrographolide and In Vitro Evaluation of Its Antiproliferative and Proapoptotic Effects on Cervical Cancer
- PMID: 33815659
- PMCID: PMC8010528
- DOI: 10.1155/2021/6692628
Inhibition of Inducible Nitric Oxide Synthase (iNOS) by Andrographolide and In Vitro Evaluation of Its Antiproliferative and Proapoptotic Effects on Cervical Cancer
Abstract
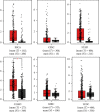
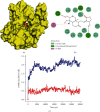
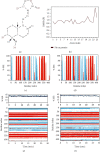
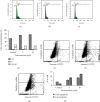
This work is aimed at investigating the expression levels of inducible nitric oxide synthase (iNOS) in cervical cancer and identifying a potential iNOS inhibitor. The data mining studies performed advocated iNOS to be a promising biomarker for cancer prognosis, as it is highly overexpressed in several malignant cancers. The elevated iNOS was found to be associated with poor survival and increased tumor aggressiveness in cervical cancer. Immunohistochemical and RT-PCR investigations of iNOS showed significant upregulation of endogenous iNOS expression in the cervical tumor samples, thus making iNOS a potent target for decreasing tumor inflammation and aggressiveness. Andrographolide, a plant-derived diterpenoid lactone, is widely reported to be effective against infections and inflammation, causing no adverse side effects on humans. In the current study, we investigated the effect of andrographolide on the prognostic value of iNOS expression in cervical cancer, which has not been reported previously. The binding efficacy of andrographolide was analyzed by performing molecular docking and molecular dynamic simulations. Multiple parameters were used to analyze the simulation trajectory, like root mean square deviation (RMSD), torsional degree of freedom, protein-root mean square fluctuations (P-RMSF), ligand RMSF, total number of intramolecular hydrogen bonds, secondary structure elements (SSE) of the protein, and protein complex with the time-dependent functions of MDS. Ligand-protein interactions revealed binding efficacy of andrographolide with tryptophan amino acid of iNOS protein. Cancer cell proliferation, cell migration, cell cycle analysis, and apoptosis-mediated cell death were assessed in vitro, post iNOS inhibition induced by andrographolide treatment (demonstrated by Western blot). Results. Andrographolide exhibited cytotoxicity by inhibiting the in vitro proliferation of cervical cancer cells and also abrogated the cancer cell migration. A significant increase in apoptosis was observed with increasing andrographolide concentration, and it also induced cell cycle arrest at G1-S phase transition. Our results substantiate that andrographolide significantly inhibits iNOS expression and exhibits antiproliferative and proapoptotic effects on cervical cancer cells.
Copyright © 2021 Akbar Pasha et al.
Conflict of interest statement
The authors declare that there is no conflict of interest. No additional benefits will be received from a third party directly or indirectly by the authors.
Figures





References
-
- Georgescu S. R., Mitran C. I., Mitran M. I., et al. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. Journal of Immunology Research . 2018;2018:10. doi: 10.1155/2018/5315816.5315816 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

