Functional maturation of immature β cells: A roadblock for stem cell therapy for type 1 diabetes
- PMID: 33815669
- PMCID: PMC8006013
- DOI: 10.4252/wjsc.v13.i3.193
Functional maturation of immature β cells: A roadblock for stem cell therapy for type 1 diabetes
Abstract
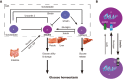
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease caused by the specific destruction of pancreatic islet β cells and is characterized as the absolute insufficiency of insulin secretion. Current insulin replacement therapy supplies insulin in a non-physiological way and is associated with devastating complications. Experimental islet transplantation therapy has been proven to restore glucose homeostasis in people with severe T1DM. However, it is restricted by many factors such as severe shortage of donor sources, progressive loss of donor cells, high cost, etc. As pluripotent stem cells have the potential to give rise to all cells including islet β cells in the body, stem cell therapy for diabetes has attracted great attention in the academic community and the general public. Transplantation of islet β-like cells differentiated from human pluripotent stem cells (hPSCs) has the potential to be an excellent alternative to islet transplantation. In stem cell therapy, obtaining β cells with complete insulin secretion in vitro is crucial. However, after much research, it has been found that the β-like cells obtained by in vitro differentiation still have many defects, including lack of adult-type glucose stimulated insulin secretion, and multi-hormonal secretion, suggesting that in vitro culture does not allows for obtaining fully mature β-like cells for transplantation. A large number of studies have found that many transcription factors play important roles in the process of transforming immature to mature human islet β cells. Furthermore, PDX1, NKX6.1, SOX9, NGN3, PAX4, etc., are important in inducing hPSC differentiation in vitro. The absent or deficient expression of any of these key factors may lead to the islet development defect in vivo and the failure of stem cells to differentiate into genuine functional β-like cells in vitro. This article reviews β cell maturation in vivo and in vitro and the vital roles of key molecules in this process, in order to explore the current problems in stem cell therapy for diabetes.
Keywords: Maturation; Stem cell therapy; Type 1 diabetes mellitus; β cell.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Figures

Similar articles
-
PDX1- /NKX6.1+ progenitors derived from human pluripotent stem cells as a novel source of insulin-secreting cells.Diabetes Metab Res Rev. 2021 Jul;37(5):e3400. doi: 10.1002/dmrr.3400. Epub 2020 Sep 2. Diabetes Metab Res Rev. 2021. PMID: 32857429
-
Human pluripotent stem cell-derived β cells: Truly immature islet β cells for type 1 diabetes therapy?World J Stem Cells. 2023 Apr 26;15(4):182-195. doi: 10.4252/wjsc.v15.i4.182. World J Stem Cells. 2023. PMID: 37180999 Free PMC article. Review.
-
Highly Efficient Differentiation of Human Pluripotent Stem Cells into Pancreatic Progenitors Co-expressing PDX1 and NKX6.1.Methods Mol Biol. 2022;2454:351-363. doi: 10.1007/7651_2020_323. Methods Mol Biol. 2022. PMID: 33190184
-
In Vivo Differentiation of Stem Cell-derived Human Pancreatic Progenitors to Treat Type 1 Diabetes.Stem Cell Rev Rep. 2020 Dec;16(6):1139-1155. doi: 10.1007/s12015-020-10018-5. Stem Cell Rev Rep. 2020. PMID: 32844324 Review.
-
Human Induced Pluripotent Stem Cells in the Curative Treatment of Diabetes and Potential Impediments Ahead.Adv Exp Med Biol. 2019;1144:25-35. doi: 10.1007/5584_2018_305. Adv Exp Med Biol. 2019. PMID: 30569414 Review.
Cited by
-
PDX1 is the cornerstone of pancreatic β-cell functions and identity.Front Mol Biosci. 2022 Dec 15;9:1091757. doi: 10.3389/fmolb.2022.1091757. eCollection 2022. Front Mol Biosci. 2022. PMID: 36589234 Free PMC article. Review.
-
The Metabolic Basis for Nervous System Dysfunction in Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease.Curr Neurovasc Res. 2023;20(3):314-333. doi: 10.2174/1567202620666230721122957. Curr Neurovasc Res. 2023. PMID: 37488757 Free PMC article.
-
Channeling β-cell Maturity: KATP Surface Localization Imparts Glucose Sensing.Endocrinology. 2021 Nov 1;162(11):bqab171. doi: 10.1210/endocr/bqab171. Endocrinology. 2021. PMID: 34402896 Free PMC article. No abstract available.
-
Photo-click hydrogels for 3D in situ differentiation of pancreatic progenitors from induced pluripotent stem cells.Stem Cell Res Ther. 2023 Aug 30;14(1):223. doi: 10.1186/s13287-023-03457-7. Stem Cell Res Ther. 2023. PMID: 37649117 Free PMC article.
-
Optogenetic therapeutic strategies for diabetes mellitus.J Diabetes. 2024 Jun;16(6):e13557. doi: 10.1111/1753-0407.13557. J Diabetes. 2024. PMID: 38751366 Free PMC article. Review.
References
-
- Cobo-Vuilleumier N, Lorenzo PI, Rodríguez NG, Herrera Gómez IG, Fuente-Martin E, López-Noriega L, Mellado-Gil JM, Romero-Zerbo SY, Baquié M, Lachaud CC, Stifter K, Perdomo G, Bugliani M, De Tata V, Bosco D, Parnaud G, Pozo D, Hmadcha A, Florido JP, Toscano MG, de Haan P, Schoonjans K, Sánchez Palazón L, Marchetti P, Schirmbeck R, Martín-Montalvo A, Meda P, Soria B, Bermúdez-Silva FJ, St-Onge L, Gauthier BR. LRH-1 agonism favours an immune-islet dialogue which protects against diabetes mellitus. Nat Commun. 2018;9:1488. - PMC - PubMed
-
- Yin L, Zeng C, Yao J, Shen J. Emerging Roles for Noncoding RNAs in Autoimmune Thyroid Disease. Endocrinology. 2020;161 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

