Electro-opening of a microtubule lattice in silico
- PMID: 33815687
- PMCID: PMC7985272
- DOI: 10.1016/j.csbj.2021.02.007
Electro-opening of a microtubule lattice in silico
Abstract
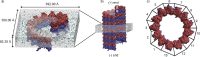
Modulation of the structure and function of biomaterials is essential for advancing bio-nanotechnology and biomedicine. Microtubules (MTs) are self-assembled protein polymers that are essential for fundamental cellular processes and key model compounds for the design of active bio-nanomaterials. In this in silico study, a 0.5 μs-long all-atom molecular dynamics simulation of a complete MT with approximately 1.2 million atoms in the system indicated that a nanosecond-scale intense electric field can induce the longitudinal opening of the cylindrical shell of the MT lattice, modifying the structure of the MT. This effect is field-strength- and temperature-dependent and occurs on the cathode side. A model was formulated to explain the opening on the cathode side, which resulted from an electric-field-induced imbalance between electric torque on tubulin dipoles and cohesive forces between tubulin heterodimers. Our results open new avenues for electromagnetic modulation of biological and artificial materials through action on noncovalent molecular interactions.
Keywords: Electric field; Microtubules; Molecular dynamics simulation; Proteins; Tubulin.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Ross T.D., Lee H.J., Qu Z., Banks R.A., Phillips R., Thomson M. Controlling organization and forces in active matter through optically defined boundaries. Nature. 2019;572:224–229. doi: 10.1038/s41586-019-1447-1. URL:http://www.nature.com/articles/s41586-019-1447-1. - DOI - PMC - PubMed
-
- Duclos G., Adkins R., Banerjee D., Peterson M.S.E., Varghese M., Kolvin I., Baskaran A., Pelcovits R.A., Powers T.R., Baskaran A., Toschi F., Hagan M.F., Streichan S.J., Vitelli V., Beller D.A., Dogic Z. Topological structure and dynamics of three-dimensional active nematics. Science. 2020;367:1120–1124. doi: 10.1126/science.aaz4547. URL:https://www.sciencemag.org/lookup/doi/10.1126/science.aaz4547. - DOI - PMC - PubMed
-
- Kellogg E.H., Hejab N.M.A., Poepsel S., Downing K.H., DiMaio F., Nogales E. Near-atomic model of microtubule-tau interactions. Science. 2018;360:1242–1246. doi: 10.1126/science.aat1780. URL:http://www.sciencemag.org/lookup/doi/10.1126/science.aat1780. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
