Correlation Between Dual-Time-Point FDG PET and Tumor Microenvironment Immune Types in Non-Small Cell Lung Cancer
- PMID: 33816219
- PMCID: PMC8012725
- DOI: 10.3389/fonc.2021.559623
Correlation Between Dual-Time-Point FDG PET and Tumor Microenvironment Immune Types in Non-Small Cell Lung Cancer
Abstract
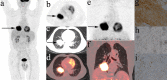
Purpose: Dual-time-point 18F-fluorodeoxyglucose positron emission tomography (DTP 18F-FDG PET), which reflects the dynamics of tumor glucose metabolism, may also provide a novel approach to the characterization of both cancer cells and immune cells within the tumor immune microenvironment (TIME). We investigated the correlations between the metabolic parameters (MPs) of DTP 18F-FDG PET images and the tumor microenvironment immune types (TMITs) in patients with non-small cell lung cancer (NSCLC).
Methods: A retrospective analysis was performed in 91 patients with NSCLC who underwent preoperative DTP 18F-FDG PET/CT scans. MPs in the early scan (eSUVmax, eSUVmean, eMTV, eTLG) and delayed scan (dSUVmax, dSUVmean, dMTV, dTLG) were calculated, respectively. The change in MPs (ΔSUVmax, ΔSUVmean, ΔMTV, ΔTLG) between the two time points were calculated. Tumor specimens were analyzed by immunohistochemistry for PD-1/PD-L1 expression and CD8+ tumor-infiltrating lymphocytes (TILs). TIME was classified into four immune types (TMIT I ~ IV) according to the expression of PD-L1 and CD8+ TILs. Correlations between MPs with TMITs and the immune-related biomarkers were analyzed. A composite metabolic signature (Meta-Sig) and a combined model of Meta-Sig and clinical factors were constructed to predict patients with TMIT I tumors.
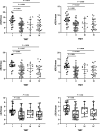
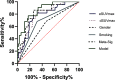
Results: eSUVmax, eSUVmean, dSUVmax, dSUVmean, ΔSUVmax, ΔSUVmean, and ΔTLG were significantly higher in PD-L1 positive patients (p = 0.0007, 0.0006, < 0.0001, < 0.0001, 0.0002, 0.0002, 0.0247, respectively), and in TMIT-I tumors (p = 0.0001, < 0.0001, < 0.0001, < 0.0001, 0.0009, 0.0009, 0.0144, respectively). Compared to stand-alone MP, the Meta-Sig and combined model displayed better performance for assessing TMIT-I tumors (Meta-sig: AUC = 0.818, sensitivity = 86.36%, specificity = 73.91%; Model: AUC = 0.869, sensitivity = 77.27%, specificity = 82.61%).
Conclusion: High glucose metabolism on DTP 18F-FDG PET correlated with the TMIT-I tumors, and the Meta-Sig and combined model based on clinical and metabolic information could improve the performance of identifying the patients who may respond to immunotherapy.
Keywords: DTP 18F-FDG PET; NSCLC; PD-L1; metabolic parameters; tumor microenvironment immune types.
Copyright © 2021 Zhou, Zou, Cheng, Kuang, Li, Chen, Liu, Yan and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A Novel Approach Using FDG-PET/CT-Based Radiomics to Assess Tumor Immune Phenotypes in Patients With Non-Small Cell Lung Cancer.Front Oncol. 2021 Nov 17;11:769272. doi: 10.3389/fonc.2021.769272. eCollection 2021. Front Oncol. 2021. PMID: 34868999 Free PMC article.
-
Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery.Eur J Nucl Med Mol Imaging. 2016 Oct;43(11):1954-61. doi: 10.1007/s00259-016-3425-2. Epub 2016 Jun 1. Eur J Nucl Med Mol Imaging. 2016. PMID: 27251642
-
Noninvasive evaluation of tumor immune microenvironment in patients with clear cell renal cell carcinoma using metabolic parameter from preoperative 2-[18F]FDG PET/CT.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):4054-4066. doi: 10.1007/s00259-021-05399-9. Epub 2021 May 12. Eur J Nucl Med Mol Imaging. 2021. PMID: 33978830
-
Predictive value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for PD-L1 expression in non-small cell lung cancer: A systematic review and meta-analysis.Thorac Cancer. 2020 Nov;11(11):3260-3268. doi: 10.1111/1759-7714.13664. Epub 2020 Sep 20. Thorac Cancer. 2020. PMID: 32951338 Free PMC article.
-
Value of 18F-FDG-PET to predict PD-L1 expression and outcomes of PD-1 inhibition therapy in human cancers.Cancer Imaging. 2021 Jan 13;21(1):11. doi: 10.1186/s40644-021-00381-y. Cancer Imaging. 2021. PMID: 33441183 Free PMC article. Review.
Cited by
-
Tumor infiltrating lymphocytes and radiological picture of the tumor.Med Oncol. 2023 May 13;40(6):176. doi: 10.1007/s12032-023-02036-3. Med Oncol. 2023. PMID: 37178270 Free PMC article. Review.
-
Metabolic characterization and radiomics-based composite model for breast cancer immune microenvironment types using 18F-FDG PET/CT.Eur J Nucl Med Mol Imaging. 2025 May 6. doi: 10.1007/s00259-025-07306-y. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40325259
-
Applications of CT-based radiomics for the prediction of immune checkpoint markers and immunotherapeutic outcomes in non-small cell lung cancer.Front Immunol. 2024 Aug 22;15:1434171. doi: 10.3389/fimmu.2024.1434171. eCollection 2024. Front Immunol. 2024. PMID: 39238640 Free PMC article. Review.
-
Prognostic Significance of Volumetric Parameters Based on FDG PET/CT in Patients with Lung Adenocarcinoma Undergoing Curative Surgery.Cancers (Basel). 2023 Sep 1;15(17):4380. doi: 10.3390/cancers15174380. Cancers (Basel). 2023. PMID: 37686654 Free PMC article.
-
Radiomic Signatures Associated with CD8+ Tumour-Infiltrating Lymphocytes: A Systematic Review and Quality Assessment Study.Cancers (Basel). 2022 Jul 27;14(15):3656. doi: 10.3390/cancers14153656. Cancers (Basel). 2022. PMID: 35954318 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

