Comparative Efficacy and Safety of Immunotherapy Alone and in Combination With Chemotherapy for Advanced Non-small Cell Lung Cancer
- PMID: 33816241
- PMCID: PMC8013714
- DOI: 10.3389/fonc.2021.611012
Comparative Efficacy and Safety of Immunotherapy Alone and in Combination With Chemotherapy for Advanced Non-small Cell Lung Cancer
Abstract
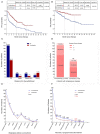
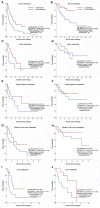
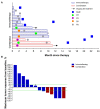
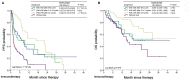
There is a lack of direct cross-comparison studies in clinical trials between immunotherapy alone and combination treatment, especially in Non-Small Cell Lung Cancer (NSCLC) patients with high PD-L1 expression. To determine if anti-PD-(L)1 antibody combined with chemotherapy is more efficient than immune checkpoint inhibitor (ICI) monotherapy for advanced NSCLC patients in the real-world data. We retrospectively collected 325 patients with advanced NSCLC treated with ICI alone with or without chemotherapy from 11th July 2016 to 26th May 2020 to investigate which treatment scenario is the most efficient, and how clinical factors impact response. Patients with advanced NSCLC were treated with ICI monotherapy (178/325, 54.8%) or in combination with chemotherapy (147/325, 45.2%). The objective response rate and disease control rate were higher in the combination group than the monotherapy group. Patients (including those with distant metastasis) treated with chemo-immunotherapy were associated with a significantly longer median PFS and OS compared with the monotherapy group, irrespective of the PD-L1 expression level and previous treatment lines. No significant increase in the risk of immune-related adverse events (irAEs) was found after combination with chemotherapy (50.6 vs. 57.8%). IrAEs predicted better PFS of immunotherapy in the monotherapy group, especially for patients with late irAEs (after ≥4 cycles). Collectively, we demonstrated that ICI monotherapy plus chemotherapy might have better anti-tumor activity and an acceptable side-effect profile regardless of PD-L1 level or previous treatment lines. Both regimens were well-tolerated and cost-effective, the more efficient is usually recommended.
Keywords: NSCLC; PD-L1; chemotherapy; immune-related adverse event; immunotherapy.
Copyright © 2021 Wang, Niu, An, Sun and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, et al. . FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. (2016) 21:634–42. 10.1634/theoncologist.2015-0507 - DOI - PMC - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819−30. 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
-
- Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. . Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. (2020) 15:1351–60. 10.1016/j.jtho.2020.03.028 - DOI - PubMed
-
- Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, et al. . Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. (2020) 21:387–97. 10.1016/S1470-2045(19)30801-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

