MicroRNA-940 as a Potential Serum Biomarker for Prostate Cancer
- PMID: 33816263
- PMCID: PMC8017318
- DOI: 10.3389/fonc.2021.628094
MicroRNA-940 as a Potential Serum Biomarker for Prostate Cancer
Abstract
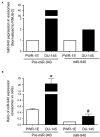
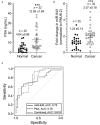
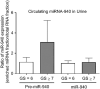
Prostate cancer is one of the leading causes of death despite an astoundingly high survival rate for localized tumors. Though prostate specific antigen (PSA) test, performed in conjunction with digital rectal examinations, is reasonably accurate, there are major caveats requiring a thorough assessment of risks and benefits prior to conducting the test. MicroRNAs, a class of small non-coding RNAs, are stable molecules that can be detected in circulation by non-invasive methods and have gained importance in cancer prognosis and diagnosis in the recent years. Here, we investigate circulating miR-940, a miRNA known to play a role in prostate cancer progression, in both cell culture supernatants as well as patient serum and urine samples to determine the utility of miR-940 as a new molecular marker for prostate cancer detection. We found that miR-940 was significantly higher in serum from cancer patients, specifically those with clinically significant tumors (GS ≥ 7). Analysis of receiver operating characteristic curve demonstrated that miR-940 in combination with PSA had a higher area under curve value (AUC: 0.818) than the miR-940 alone (AUC: 0.75) for the diagnosis of prostate cancer. This study provides promising results suggesting the use of miR-940 for prostate cancer diagnosis.
Keywords: PSA; circulating miRNA; miR-940; prostate cancer; serum.
Copyright © 2021 Rajendiran, Maji, Haddad, Lotan, Nandy, Vishwanatha and Chaudhary.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

