Peritoneal Metastases From Colorectal Cancer: Defining and Addressing the Challenges
- PMID: 33816304
- PMCID: PMC8010649
- DOI: 10.3389/fonc.2021.650098
Peritoneal Metastases From Colorectal Cancer: Defining and Addressing the Challenges
Abstract
The presence of peritoneal metastases (PM) in patients with colorectal cancer (CRC) is associated with an extremely poor prognosis. The diagnosis of PM is challenging, resulting in an underestimation of their true incidence. While surgery can be curative in a small percentage of patients, effective treatment for non-operable PM is lacking, and clinical and pre-clinical studies are relatively sparse. Here we have defined the major clinical challenges in the areas of risk assessment, detection, and treatment. Recent developments in the field include the application of organoid technology, which has generated highly relevant pre-clinical PM models, the application of diffusion-weighted MRI, which has greatly improved PM detection, and the design of small clinical proof-of-concept studies, which allows the efficient testing of new treatment strategies. Together, these developments set the stage for starting to address the clinical challenges. To help structure these efforts, a translational research framework is presented, in which clinical trial design is based on the insight gained from direct tissue analyses and pre-clinical (organoid) models derived from CRC patients with PM. This feed-forward approach, in which a thorough understanding of the disease drives innovation in its clinical management, has the potential to improve outcome in the years to come.
Keywords: CMS4; colorectal; imaging; organoid; peritoneal.
Copyright © 2021 Kranenburg, Speeten and Hingh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
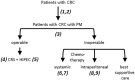
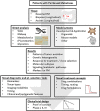
References
-
- Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. . Research in cancers of the digestive system: prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. (2016) 17:1709–19. 10.1016/S144444470-2045(16)30500-9 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

