The Limitless Future of RNA Therapeutics
- PMID: 33816449
- PMCID: PMC8012680
- DOI: 10.3389/fbioe.2021.628137
The Limitless Future of RNA Therapeutics
Abstract
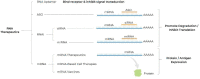
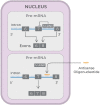
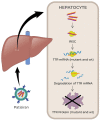
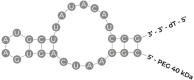
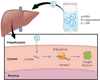
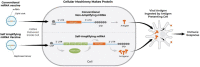
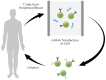
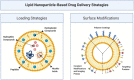
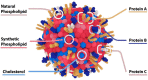
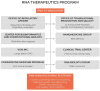
Recent advances in the generation, purification and cellular delivery of RNA have enabled development of RNA-based therapeutics for a broad array of applications. RNA therapeutics comprise a rapidly expanding category of drugs that will change the standard of care for many diseases and actualize personalized medicine. These drugs are cost effective, relatively simple to manufacture, and can target previously undruggable pathways. It is a disruptive therapeutic technology, as small biotech startups, as well as academic groups, can rapidly develop new and personalized RNA constructs. In this review we discuss general concepts of different classes of RNA-based therapeutics, including antisense oligonucleotides, aptamers, small interfering RNAs, microRNAs, and messenger RNA. Furthermore, we provide an overview of the RNA-based therapies that are currently being evaluated in clinical trials or have already received regulatory approval. The challenges and advantages associated with use of RNA-based drugs are also discussed along with various approaches for RNA delivery. In addition, we introduce a new concept of hospital-based RNA therapeutics and share our experience with establishing such a platform at Houston Methodist Hospital.
Keywords: RNA therapeutics; delivery of RNA therapeutics; hospital-based RNA therapeutics; messenger RNAs (mRNAs); self-amplifying mRNA.
Copyright © 2021 Damase, Sukhovershin, Boada, Taraballi, Pettigrew and Cooke.
Conflict of interest statement
Houston Methodist Hospital has been assigned intellectual property related to the synthesis, purification, validation, and delivery of nucleic acid therapeutics. JPC is an inventor on issued patents related to mRNA telomerase therapy, which have been assigned to Stanford University and licensed to his company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alnylam (2020a). Alnylam® Development Pipeline of Investigational RNAi Therapeutics Alnylam. Available online at: https://www.alnylam.com/alnylam-rnai-pipeline/ (accessed January 3, 2020).
-
- Alnylam (2020b). Alnylam Announces Approval of GIVLAARITM (givosiran) by the U.S. Food and Drug Administration (FDA) Alnylam Pharm. Inc. Available online at: http://investors.alnylam.com/news-releases/news-release-details/alnylam-... (accessed January 3, 2020).
-
- Amgen (2020). FDA Approves IMLYGIC Talimogene Laherparepvec As First Oncolytic Viral Therapy In The US Amgen Inc. Available online at: http://www.amgen.com/en/media/news-releases/2015/10/fda-approves-imlygic... (accessed August 18, 2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

