Spatial Learning Is Impaired in Male Pubertal Rats Following Neonatal Daily but Not Randomly Spaced Maternal Deprivation
- PMID: 33816470
- PMCID: PMC8012507
- DOI: 10.3389/fcell.2021.621308
Spatial Learning Is Impaired in Male Pubertal Rats Following Neonatal Daily but Not Randomly Spaced Maternal Deprivation
Abstract
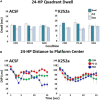
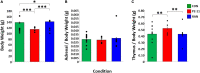
Severe early life stress has long been associated with neuropsychological disorders in adulthood, including depression, schizophrenia, post-traumatic stress disorder, and memory dysfunction. To some extent, all of these conditions involve dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and reduced negative feedback inhibition of cortisol release in adulthood. However, the time course for mental health and hormonal outcomes across life stages and the attributes of early life stress that direct the behavioral and biological alterations is not fully understood. We designed our studies to compare outcomes of the two most common maternal deprivation schedules on cognitive ability prior to adulthood. We exposed rat pups to daily or randomly spaced maternal separation bouts within the first 3 weeks of life and examined cognitive performance, neurotrophic signaling, and stress and immune system markers during puberty. We found that the daily separation schedule impaired spatial learning while the randomly spaced schedule did not alter maze performance relative to normally reared control animals. Animals that underwent daily separation showed a tendency for reduced body weight compared to the randomly spaced condition, but there were no differences in adrenal weight. Thymus weight normalized by body weight was increased following daily separation compared to random separation and control conditions. Plasma corticosterone levels measured after behavior testing did not differ amongst experimental groups and there was no impact of TrKB receptor inhibition. Combined, the results show that different early life stress schedules produce different behavioral and biological outcomes when measured at puberty. Combined with prior findings from more mature animals, the results presented here suggest that daily neonatal stress produces varied alterations in spatial cognition at different life stages with a transient learning deficit at puberty preceding a more persistent and a progressive memory impairment through adulthood and into aging.
Keywords: brain derived neuronal factor; corticosterone; glucocorticoid receptor; hippocampus; maternal deprivation; spatial learning; tyrosine receptor kinase B.
Copyright © 2021 Stoneham, McHail, Samipour-Biel, Liehr, Lee, Evans, Boggs and Dumas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


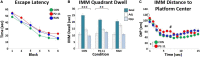


Similar articles
-
Maternal separation blunted spatial memory formation independent of peripheral and hippocampal insulin content in young adult male rats.PLoS One. 2018 Oct 17;13(10):e0204731. doi: 10.1371/journal.pone.0204731. eCollection 2018. PLoS One. 2018. PMID: 30332425 Free PMC article.
-
Early maternal deprivation reduces prepulse inhibition and impairs spatial learning ability in adulthood: no further effect of post-pubertal chronic corticosterone treatment.Behav Brain Res. 2007 Jan 25;176(2):323-32. doi: 10.1016/j.bbr.2006.10.020. Epub 2006 Nov 9. Behav Brain Res. 2007. PMID: 17097157
-
Maternal separation modulates short-term behavioral and physiological indices of the stress response.Horm Behav. 2010 Jul;58(2):241-9. doi: 10.1016/j.yhbeh.2010.03.010. Epub 2010 Mar 15. Horm Behav. 2010. PMID: 20298695
-
The stress system in the human brain in depression and neurodegeneration.Ageing Res Rev. 2005 May;4(2):141-94. doi: 10.1016/j.arr.2005.03.003. Ageing Res Rev. 2005. PMID: 15996533 Review.
-
Do early-life events permanently alter behavioral and hormonal responses to stressors?Int J Dev Neurosci. 1998 Jun-Jul;16(3-4):149-64. doi: 10.1016/s0736-5748(98)00025-2. Int J Dev Neurosci. 1998. PMID: 9785112 Review.
Cited by
-
Sex-specific behavioral outcomes of early-life adversity and emerging microglia-dependent mechanisms.Front Behav Neurosci. 2022 Oct 4;16:1013865. doi: 10.3389/fnbeh.2022.1013865. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36268470 Free PMC article.
-
Commercial hatchery practices have long-lasting effects on laying hens' spatial behaviour and health.PLoS One. 2023 Dec 20;18(12):e0295560. doi: 10.1371/journal.pone.0295560. eCollection 2023. PLoS One. 2023. PMID: 38117840 Free PMC article.
-
Comparing the effects of infant maternal and sibling separation on adolescent behavior in rats (Rattus norvegicus).PLoS One. 2024 Aug 16;19(8):e0308958. doi: 10.1371/journal.pone.0308958. eCollection 2024. PLoS One. 2024. PMID: 39150925 Free PMC article.
-
Optogenetic modulation of hippocampal oscillations ameliorates spatial cognition and hippocampal dysrhythmia following early-life seizures.Neurobiol Dis. 2023 Mar;178:106021. doi: 10.1016/j.nbd.2023.106021. Epub 2023 Jan 28. Neurobiol Dis. 2023. PMID: 36720444 Free PMC article.
-
Antidepressant Shugan Jieyu Capsule Alters Gut Microbiota and Intestinal Microbiome Function in Rats With Chronic Unpredictable Mild Stress -Induced Depression.Front Pharmacol. 2022 Jun 13;13:828595. doi: 10.3389/fphar.2022.828595. eCollection 2022. Front Pharmacol. 2022. PMID: 35770090 Free PMC article.
References
-
- Aisa B., Tordera R., Lasheras B., Del Rio J., Ramirez M. J. (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32 256–266. - PubMed
-
- Anisman H., Zaharia M. D., Meaney M. J., Merali Z. (1998). Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 16 149–164. - PubMed
-
- Berry B., McMahan R., Gallagher M. (1997). Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav. Neurosci. 111 267–274. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

