Different Effects of Intramedullary Injection of Mesenchymal Stem Cells During the Acute vs. Chronic Inflammatory Phase on Bone Healing in the Murine Continuous Polyethylene Particle Infusion Model
- PMID: 33816480
- PMCID: PMC8017138
- DOI: 10.3389/fcell.2021.631063
Different Effects of Intramedullary Injection of Mesenchymal Stem Cells During the Acute vs. Chronic Inflammatory Phase on Bone Healing in the Murine Continuous Polyethylene Particle Infusion Model
Abstract
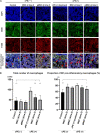
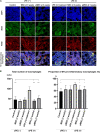
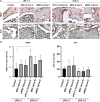
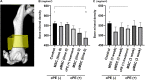
Chronic inflammation is a common feature in many diseases of different organ systems, including bone. However, there are few interventions to mitigate chronic inflammation and preserve host tissue. Previous in vitro studies demonstrated that preconditioning of mesenchymal stem cells (pMSCs) using lipopolysaccharide and tumor necrosis factor-α polarized macrophages from a pro-inflammatory to an anti-inflammatory phenotype and increased osteogenesis compared to unaltered MSCs. In the current study, we investigated the local injection of MSCs or pMSCs during the acute versus chronic inflammatory phase in a murine model of inflammation of bone: the continuous femoral intramedullary polyethylene particle infusion model. Chronic inflammation due to contaminated polyethylene particles decreased bone mineral density and increased osteoclast-like cells positively stained with leukocyte tartrate resistant acid phosphatase (TRAP) staining, and resulted in a sustained M1 pro-inflammatory macrophage phenotype and a decreased M2 anti-inflammatory phenotype. Local injection of MSCs or pMSCs during the chronic inflammatory phase reversed these findings. Conversely, immediate local injection of pMSCs during the acute inflammatory phase impaired bone healing, probably by mitigating the mandatory acute inflammatory reaction. These results suggest that the timing of interventions to facilitate bone healing by modulating inflammation is critical to the outcome. Interventions to facilitate bone healing by modulating acute inflammation should be prudently applied, as this phase of bone healing is temporally sensitive. Alternatively, local injection of MSCs or pMSCs during the chronic inflammatory phase may be a potential intervention to mitigate the adverse effects of contaminated particles on bone.
Keywords: bone healing; inflammation; macrophage; mesenchymal stem cell; osteolysis.
Copyright © 2021 Utsunomiya, Zhang, Lin, Kohno, Ueno, Maruyama, Rhee, Huang, Yao and Goodman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Sex differences in the therapeutic effect of unaltered versus NFκB sensing IL-4 over-expressing mesenchymal stromal cells in a murine model of chronic inflammatory bone loss.Front Bioeng Biotechnol. 2022 Aug 15;10:962114. doi: 10.3389/fbioe.2022.962114. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36046680 Free PMC article.
-
Therapeutic effects of MSCs, genetically modified MSCs, and NFĸB-inhibitor on chronic inflammatory osteolysis in aged mice.J Orthop Res. 2023 May;41(5):1004-1013. doi: 10.1002/jor.25434. Epub 2022 Sep 8. J Orthop Res. 2023. PMID: 36031590 Free PMC article.
-
Mesenchymal Stem Cells and NF-κB Sensing Interleukin-4 Over-Expressing Mesenchymal Stem Cells Are Equally Effective in Mitigating Particle-Associated Chronic Inflammatory Bone Loss in Mice.Front Cell Dev Biol. 2021 Oct 14;9:757830. doi: 10.3389/fcell.2021.757830. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34722543 Free PMC article.
-
Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy.Inflamm Regen. 2023 May 25;43(1):29. doi: 10.1186/s41232-023-00279-1. Inflamm Regen. 2023. PMID: 37231450 Free PMC article. Review.
-
Inflammation and Bone Repair: From Particle Disease to Tissue Regeneration.Front Bioeng Biotechnol. 2019 Sep 19;7:230. doi: 10.3389/fbioe.2019.00230. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31608274 Free PMC article. Review.
Cited by
-
Sex differences in the therapeutic effect of unaltered versus NFκB sensing IL-4 over-expressing mesenchymal stromal cells in a murine model of chronic inflammatory bone loss.Front Bioeng Biotechnol. 2022 Aug 15;10:962114. doi: 10.3389/fbioe.2022.962114. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36046680 Free PMC article.
-
Ageing attenuates bone healing by mesenchymal stem cells in a microribbon hydrogel with a murine long bone critical-size defect model.Immun Ageing. 2022 Mar 12;19(1):14. doi: 10.1186/s12979-022-00272-1. Immun Ageing. 2022. PMID: 35279175 Free PMC article.
-
Nanoparticles and bone microenvironment: a comprehensive review for malignant bone tumor diagnosis and treatment.Mol Cancer. 2024 Nov 1;23(1):246. doi: 10.1186/s12943-024-02161-1. Mol Cancer. 2024. PMID: 39487487 Free PMC article. Review.
-
Therapeutic effects of MSCs, genetically modified MSCs, and NFĸB-inhibitor on chronic inflammatory osteolysis in aged mice.J Orthop Res. 2023 May;41(5):1004-1013. doi: 10.1002/jor.25434. Epub 2022 Sep 8. J Orthop Res. 2023. PMID: 36031590 Free PMC article.
-
The efficacy of lapine preconditioned or genetically modified IL4 over-expressing bone marrow-derived mesenchymal stromal cells in corticosteroid-associated osteonecrosis of the femoral head in rabbits.Biomaterials. 2021 Aug;275:120972. doi: 10.1016/j.biomaterials.2021.120972. Epub 2021 Jun 21. Biomaterials. 2021. PMID: 34186237 Free PMC article.
References
-
- Aktas E., Chamberlain C. S., Seather E. E., Duenwald-Kuehi S. E., Kondratko-Mittnacht J., Stitgen M., et al. (2017). Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J. Orthop. Res. 35 269–280. 10.1002/stem.1040 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

