Combination of Angiotensin (1-7) Agonists and Convalescent Plasma as a New Strategy to Overcome Angiotensin Converting Enzyme 2 (ACE2) Inhibition for the Treatment of COVID-19
- PMID: 33816521
- PMCID: PMC8012486
- DOI: 10.3389/fmed.2021.620990
Combination of Angiotensin (1-7) Agonists and Convalescent Plasma as a New Strategy to Overcome Angiotensin Converting Enzyme 2 (ACE2) Inhibition for the Treatment of COVID-19
Abstract
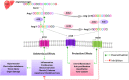
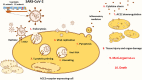
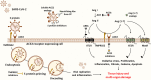
Coronavirus disease-2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently the most concerning health problem worldwide. SARS-CoV-2 infects cells by binding to angiotensin-converting enzyme 2 (ACE2). It is believed that the differential response to SARS-CoV-2 is correlated with the differential expression of ACE2. Several reports proposed the use of ACE2 pharmacological inhibitors and ACE2 antibodies to block viral entry. However, ACE2 inhibition is associated with lung and cardiovascular pathology and would probably increase the pathogenesis of COVID-19. Therefore, utilizing ACE2 soluble analogs to block viral entry while rescuing ACE2 activity has been proposed. Despite their protective effects, such analogs can form a circulating reservoir of the virus, thus accelerating its spread in the body. Levels of ACE2 are reduced following viral infection, possibly due to increased viral entry and lysis of ACE2 positive cells. Downregulation of ACE2/Ang (1-7) axis is associated with Ang II upregulation. Of note, while Ang (1-7) exerts protective effects on the lung and cardiovasculature, Ang II elicits pro-inflammatory and pro-fibrotic detrimental effects by binding to the angiotensin type 1 receptor (AT1R). Indeed, AT1R blockers (ARBs) can alleviate the harmful effects associated with Ang II upregulation while increasing ACE2 expression and thus the risk of viral infection. Therefore, Ang (1-7) agonists seem to be a better treatment option. Another approach is the transfusion of convalescent plasma from recovered patients with deteriorated symptoms. Indeed, this appears to be promising due to the neutralizing capacity of anti-COVID-19 antibodies. In light of these considerations, we encourage the adoption of Ang (1-7) agonists and convalescent plasma conjugated therapy for the treatment of COVID-19 patients. This therapeutic regimen is expected to be a safer choice since it possesses the proven ability to neutralize the virus while ensuring lung and cardiovascular protection through modulation of the inflammatory response.
Keywords: ACE2; Angiotensin 1-7 (Ang1-7); COVID-19; SARS-CoV-2; cardiovascular pathology; combination therapy; convalescent plasma (CP); lung pathology.
Copyright © 2021 Issa, Eid, Berry, Takhviji, Khosravi, Mantash, Nehme, Hallal, Karaki, Dhayni, Faour, Kobeissy, Nehme and Zibara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

