Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma
- PMID: 33816783
- PMCID: PMC7985479
- DOI: 10.1016/j.omto.2021.03.001
Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma
Abstract
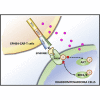
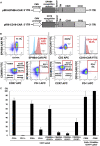
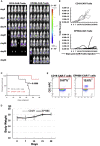
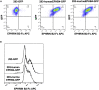
Ephrin type-B receptor 4 (EPHB4), expressed in tumors including rhabdomyosarcoma, is a suitable target for chimeric antigen receptor (CAR)-T cells. Ligand-independent activation of EPHB4 causes cell proliferation and malignant transformation in rhabdomyosarcoma, whereas ligand-dependent stimulation of EPHB4 induces apoptosis in rhabdomyosarcoma. Therefore, we hypothesized that ligand-based, EPHB4-specific CAR-T cells may kill rhabdomyosarcoma cells without stimulating downstream cell proliferation mechanisms. We developed novel CAR-T cells by targeting EPHB4 via EPHRIN B2, a natural ligand of EPHB4. The generation of EPHB4-CAR-T cells via piggyBac (PB) transposon-based gene transfer resulted in sufficient T cell expansion and CAR positivity (78.5% ± 5.9%). PB-EPHB4-CAR-T cells displayed a dominant stem cell memory fraction (59.4% ± 7.2%) as well as low PD-1 expression (0.60% ± 0.21%) after 14 days of expansion. The PB-EPHB4-CAR-T cells inhibited EPHB4-positive tumor cells without activating cell proliferation downstream of EPHB4, even after multiple tumor re-challenges and suppressed tumor growth in xenograft-bearing mice. Therefore, PB-EPHB4-CAR-T cells possess a memory-rich fraction without early T cell exhaustion and show potential as promising therapeutic agents for treating rhabdomyosarcoma and other EPHB4-positive tumors.
Keywords: CAR-T cell therapy; EPHB4; chimeric antigen receptor; piggyBac transposon; rhabdomyosarcoma; stem cell memory-like T cells.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. - PMC - PubMed
-
- Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

