European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke
- PMID: 33817340
- PMCID: PMC7995316
- DOI: 10.1177/2396987321989865
European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke
Abstract
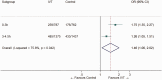
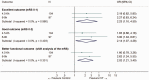
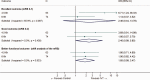
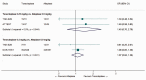
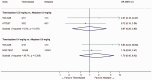
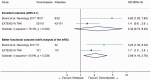
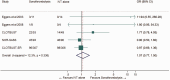
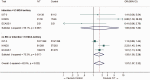
Intravenous thrombolysis is the only approved systemic reperfusion treatment for patients with acute ischaemic stroke. These European Stroke Organisation (ESO) guidelines provide evidence-based recommendations to assist physicians in their clinical decisions with regard to intravenous thrombolysis for acute ischaemic stroke. These guidelines were developed based on the ESO standard operating procedure and followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. The working group identified relevant clinical questions, performed systematic reviews and meta-analyses of the literature, assessed the quality of the available evidence, and wrote recommendations. Expert consensus statements were provided if not enough evidence was available to provide recommendations based on the GRADE approach. We found high quality evidence to recommend intravenous thrombolysis with alteplase to improve functional outcome in patients with acute ischemic stroke within 4.5 h after symptom onset. We also found high quality evidence to recommend intravenous thrombolysis with alteplase in patients with acute ischaemic stroke on awakening from sleep, who were last seen well more than 4.5 h earlier, who have MRI DWI-FLAIR mismatch, and for whom mechanical thrombectomy is not planned. These guidelines provide further recommendations regarding patient subgroups, late time windows, imaging selection strategies, relative and absolute contraindications to alteplase, and tenecteplase. Intravenous thrombolysis remains a cornerstone of acute stroke management. Appropriate patient selection and timely treatment are crucial. Further randomized controlled clinical trials are needed to inform clinical decision-making with regard to tenecteplase and the use of intravenous thrombolysis before mechanical thrombectomy in patients with large vessel occlusion.
Keywords: Ischaemic stroke; fibrinolysis; recommendations; thrombectomy; thrombolysis.
© European Stroke Organisation 2021.
Conflict of interest statement
Declaration of conflicting interests: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All disclosures are listed in Supplemental Table 1
Figures

Similar articles
-
European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke.Eur Stroke J. 2023 Mar;8(1):8-54. doi: 10.1177/23969873221150022. Epub 2023 Feb 2. Eur Stroke J. 2023. PMID: 37021186 Free PMC article.
-
European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE).Eur Stroke J. 2019 Mar;4(1):6-12. doi: 10.1177/2396987319832140. Epub 2019 Feb 26. Eur Stroke J. 2019. PMID: 31165090 Free PMC article.
-
European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke.J Neurointerv Surg. 2019 Jun;11(6):535-538. doi: 10.1136/neurintsurg-2018-014568. J Neurointerv Surg. 2019. PMID: 31152058
-
European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke.Eur Stroke J. 2024 Mar;9(1):5-68. doi: 10.1177/23969873231219416. Epub 2024 Feb 21. Eur Stroke J. 2024. PMID: 38380638 Free PMC article.
-
The Changing Landscape of Intravenous Thrombolysis for Acute Ischaemic Stroke.J Clin Med. 2024 Sep 29;13(19):5826. doi: 10.3390/jcm13195826. J Clin Med. 2024. PMID: 39407885 Free PMC article. Review.
Cited by
-
Translational Medicine in Acute Ischemic Stroke and Traumatic Brain Injury-NeuroAiD Trials, from Traditional Beliefs to Evidence-Based Therapy.Biomolecules. 2024 Jun 11;14(6):680. doi: 10.3390/biom14060680. Biomolecules. 2024. PMID: 38927083 Free PMC article. Review.
-
Successful thrombolytic therapy is associated with increased granulocyte CD15 expression and reduced stroke-induced immunosuppression.Brain Behav. 2022 Oct;12(10):e2732. doi: 10.1002/brb3.2732. Epub 2022 Sep 16. Brain Behav. 2022. PMID: 36111748 Free PMC article.
-
Microwave-induced thermoacoustic imaging for the early detection of canine intracerebral hemorrhage.Front Physiol. 2022 Nov 16;13:1067948. doi: 10.3389/fphys.2022.1067948. eCollection 2022. Front Physiol. 2022. PMID: 36467679 Free PMC article.
-
Thrombolysis with Recombinant Human Prourokinase 4.5-6 h After Acute Ischemic Stroke: A Phase IIa, Randomized, and Open-Label Multicenter Clinical Trial.CNS Drugs. 2024 Jan;38(1):67-75. doi: 10.1007/s40263-023-01051-2. Epub 2023 Nov 29. CNS Drugs. 2024. PMID: 38030867 Free PMC article. Clinical Trial.
-
Thrombolytic Treatment in Wake-Up Stroke: A Propensity Score-Matched Analysis of Treatment Effectiveness in the Norwegian Stroke Registry.J Am Heart Assoc. 2024 Feb 6;13(3):e032309. doi: 10.1161/JAHA.123.032309. Epub 2024 Jan 31. J Am Heart Assoc. 2024. PMID: 38293909 Free PMC article.
References
-
- European Stroke Organisation. Guidelines for management of ischaemic stroke and transient ischaemic attack. Cerebrovasc Dis 2008; 25: 457–507. - PubMed
-
- Hart JT. The inverse care law. Lancet 1971; 1: 405–412. - PubMed
-
- Ntaios G, Bornstein NM, Caso V, et al..; European Stroke Organisation. The European stroke organisation guidelines: a standard operating procedure. Int J Stroke 2015; 10 Suppl A100: 128–135. - PubMed
-
- Guyatt GH, Oxman AD, Schunemann HJ, et al.. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol 2011; 64: 380–382. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

