Engineering precision therapies: lessons and motivations from the clinic
- PMID: 33817342
- PMCID: PMC7998714
- DOI: 10.1093/synbio/ysaa024
Engineering precision therapies: lessons and motivations from the clinic
Abstract
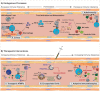
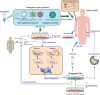
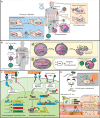
In the past decade, gene- and cell-based therapies have been at the forefront of the biomedical revolution. Synthetic biology, the engineering discipline of building sophisticated 'genetic software' to enable precise regulation of gene activities in living cells, has been a decisive success factor of these new therapies. Here, we discuss the core technologies and treatment strategies that have already gained approval for therapeutic applications in humans. We also review promising preclinical work that could either enhance the efficacy of existing treatment strategies or pave the way for new precision medicines to treat currently intractable human conditions.
Keywords: ATMP; cell-based therapies; gene therapy; synthetic biology; translational medicine.
© The Author(s) 2020. Published by Oxford University Press.
Figures

References
-
- Bailey S.R., Maus M.V. (2019) Gene editing for immune cell therapies. Nat. Biotechnol., 37, 1425–1434. - PubMed
-
- Ma C.C., Wang Z.L., Xu T., He Z.Y., Wei Y.Q. (2019) The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol. Adv., 107502. - PubMed
-
- Naldini L. (2015) Gene therapy returns to centre stage. Nature, 526, 351–360. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
