Biomimetic Ti-6Al-4V alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration
- PMID: 33817419
- PMCID: PMC7988351
- DOI: 10.1016/j.bioactmat.2021.03.010
Biomimetic Ti-6Al-4V alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration
Abstract
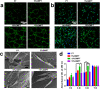
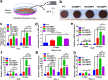
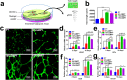
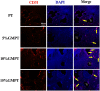
Titanium-based scaffolds are widely used implant materials for bone defect treatment. However, the unmatched biomechanics and poor bioactivities of conventional titanium-based implants usually lead to insufficient bone integration. To tackle these challenges, it is critical to develop novel titanium-based scaffolds that meet the bioadaptive requirements for load-bearing critical bone defects. Herein, inspired by the microstructure and mechanical properties of natural bone tissue, we developed a Ti-6Al-4V alloy (TC4)/gelatin methacrylate (GelMA) hybrid scaffold with dual bionic features (GMPT) for bone defect repair. GMPT is composed of a hard 3D-printed porous TC4 metal scaffold (PT) backbone, which mimics the microstructure and mechanical properties of natural cancellous bone, and a soft GelMA hydrogel matrix infiltrated into the pores of PT that mimics the microenvironment of the extracellular matrix. Ascribed to the unique dual bionic design, the resultant GMPT demonstrates better osteogenic and angiogenic capabilities than PT, as confirmed by the in vitro and rabbit radius bone defect experimental results. Moreover, controlling the concentration of GelMA (10%) in GMPT can further improve the osteogenesis and angiogenesis of GMPT. The fundamental mechanisms were revealed by RNA-Seq analysis, which showed that the concentration of GelMA significantly influenced the expression of osteogenesis- and angiogenesis-related genes via the Pi3K/Akt/mTOR pathway. The results of this work indicate that our dual bionic implant design represents a promising strategy for the restoration of large bone defects.
Keywords: 3D printing porous titanium alloys; Angiogenesis; Gelatin methacrylate; Osteogenesis.
© 2021 The Authors.
Figures






Similar articles
-
The combination of a 3D-Printed porous Ti-6Al-4V alloy scaffold and stem cell sheet technology for the construction of biomimetic engineered bone at an ectopic site.Mater Today Bio. 2022 Sep 15;16:100433. doi: 10.1016/j.mtbio.2022.100433. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36157052 Free PMC article.
-
3D-Printed Titanium Trabecular Scaffolds with Sustained Release of Hypoxia-Induced Exosomes for Dual-Mimetic Bone Regeneration.Adv Sci (Weinh). 2025 Jun;12(23):e2500599. doi: 10.1002/advs.202500599. Epub 2025 May 11. Adv Sci (Weinh). 2025. PMID: 40349160 Free PMC article.
-
Integrating 3D Printing and Biomimetic Mineralization for Personalized Enhanced Osteogenesis, Angiogenesis, and Osteointegration.ACS Appl Mater Interfaces. 2018 Dec 12;10(49):42146-42154. doi: 10.1021/acsami.8b17495. Epub 2018 Dec 3. ACS Appl Mater Interfaces. 2018. PMID: 30507136 Free PMC article.
-
GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances.Biomater Res. 2023 Sep 15;27(1):86. doi: 10.1186/s40824-023-00422-6. Biomater Res. 2023. PMID: 37715230 Free PMC article. Review.
-
Comparative analysis of corrosion resistance between beta titanium and Ti-6Al-4V alloys: A systematic review.J Trace Elem Med Biol. 2020 Dec;62:126618. doi: 10.1016/j.jtemb.2020.126618. Epub 2020 Jul 9. J Trace Elem Med Biol. 2020. PMID: 32663743
Cited by
-
Treating critical bone defects by using core-shell biological scaffold to regulate Fibrosis-Osteogenic homeostasis.Mater Today Bio. 2025 Feb 17;31:101560. doi: 10.1016/j.mtbio.2025.101560. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40083837 Free PMC article.
-
Recent progress in functional metal-organic frameworks for bio-medical application.Regen Biomater. 2023 Dec 23;11:rbad115. doi: 10.1093/rb/rbad115. eCollection 2024. Regen Biomater. 2023. PMID: 38313824 Free PMC article. Review.
-
Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment.Theranostics. 2023 Mar 27;13(6):2015-2039. doi: 10.7150/thno.80615. eCollection 2023. Theranostics. 2023. PMID: 37064871 Free PMC article.
-
The combination of a 3D-Printed porous Ti-6Al-4V alloy scaffold and stem cell sheet technology for the construction of biomimetic engineered bone at an ectopic site.Mater Today Bio. 2022 Sep 15;16:100433. doi: 10.1016/j.mtbio.2022.100433. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36157052 Free PMC article.
-
3D-printed porous Ti6Al4V scaffolds for long bone repair in animal models: a systematic review.J Orthop Surg Res. 2022 Feb 2;17(1):68. doi: 10.1186/s13018-022-02960-6. J Orthop Surg Res. 2022. PMID: 35109907 Free PMC article.
References
-
- Petite H., Viateau V., Bensaïd W., Meunier A., Pollak C.D., Bourguignon M., et al. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000;18:959. - PubMed
-
- Biggemann J., Pezoldt M., Stumpf M., Greil P., Fey T. Modular ceramic scaffolds for individual implants. Acta Biomater. 2018;80:390–400. - PubMed
-
- Reichert J.C., Cipitria A., Epari D.R., Saifzadeh S., Krishnakanth P., Berner A., et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012;4:141ra93. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

