Hypoxia-mimicking 3D bioglass-nanoclay scaffolds promote endogenous bone regeneration
- PMID: 33817422
- PMCID: PMC7988349
- DOI: 10.1016/j.bioactmat.2021.03.011
Hypoxia-mimicking 3D bioglass-nanoclay scaffolds promote endogenous bone regeneration
Abstract
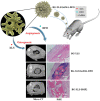
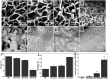
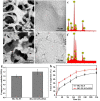
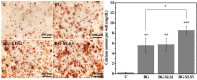
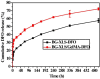
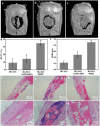
Large bone defect repair requires biomaterials that promote angiogenesis and osteogenesis. In present work, a nanoclay (Laponite, XLS)-functionalized 3D bioglass (BG) scaffold with hypoxia mimicking property was prepared by foam replication coupled with UV photopolymerization methods. Our data revealed that the incorporation of XLS can significantly promote the mechanical property of the scaffold and the osteogenic differentiation of human adipose mesenchymal stem cells (ADSCs) compared to the properties of the neat BG scaffold. Desferoxamine, a hypoxia mimicking agent, encourages bone regeneration via activating hypoxia-inducible factor-1 alpha (HIF-1α)-mediated angiogenesis. GelMA-DFO immobilization onto BG-XLS scaffold achieved sustained DFO release and inhibited DFO degradation. Furthermore, in vitro data demonstrated increased HIF-1α and vascular endothelial growth factor (VEGF) expressions on human adipose mesenchymal stem cells (ADSCs). Moreover, BG-XLS/GelMA-DFO scaffolds also significantly promoted the osteogenic differentiation of ADSCs. Most importantly, our in vivo data indicated BG-XLS/GelMA-DFO scaffolds strongly increased bone healing in a critical-sized mouse cranial bone defect model. Therefore, we developed a novel BG-XLS/GelMA-DFO scaffold which can not only induce the expression of VEGF, but also promote osteogenic differentiation of ADSCs to promote endogenous bone regeneration.
Keywords: 3D bioglass scaffold; Angiogenesis; Endogenous bone regeneration; Hypoxia; Osteogenesis.
© 2021 The Authors.
Figures







Similar articles
-
Hypoxia-Mimicking Nanofibrous Scaffolds Promote Endogenous Bone Regeneration.ACS Appl Mater Interfaces. 2016 Nov 30;8(47):32450-32459. doi: 10.1021/acsami.6b10538. Epub 2016 Nov 17. ACS Appl Mater Interfaces. 2016. PMID: 27809470 Free PMC article.
-
Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis.J Biomed Mater Res A. 2016 Oct;104(10):2515-27. doi: 10.1002/jbm.a.35793. Epub 2016 Jun 6. J Biomed Mater Res A. 2016. PMID: 27227768
-
An Electrospun DFO-Loaded Microsphere/SAIB System Orchestrates Angiogenesis-Osteogenesis Coupling via HIF-1α Activation for Vascularized Bone Regeneration.Polymers (Basel). 2025 May 31;17(11):1538. doi: 10.3390/polym17111538. Polymers (Basel). 2025. PMID: 40508781 Free PMC article.
-
Small Extracellular Vesicles Released from Bioglass/Hydrogel Scaffold Promote Vascularized Bone Regeneration by Transferring miR-23a-3p.Int J Nanomedicine. 2022 Dec 9;17:6201-6220. doi: 10.2147/IJN.S389471. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36531118 Free PMC article.
-
Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering.J Control Release. 2018 Jun 10;279:69-78. doi: 10.1016/j.jconrel.2018.04.011. Epub 2018 Apr 9. J Control Release. 2018. PMID: 29649529 Free PMC article.
Cited by
-
The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair.Pharmaceutics. 2023 Mar 22;15(3):1017. doi: 10.3390/pharmaceutics15031017. Pharmaceutics. 2023. PMID: 36986877 Free PMC article. Review.
-
Unraveling the potential of 3D bioprinted immunomodulatory materials for regulating macrophage polarization: State-of-the-art in bone and associated tissue regeneration.Bioact Mater. 2023 Jun 1;28:284-310. doi: 10.1016/j.bioactmat.2023.05.014. eCollection 2023 Oct. Bioact Mater. 2023. PMID: 37303852 Free PMC article. Review.
-
The Role of HIF-1α in Bone Regeneration: A New Direction and Challenge in Bone Tissue Engineering.Int J Mol Sci. 2023 Apr 28;24(9):8029. doi: 10.3390/ijms24098029. Int J Mol Sci. 2023. PMID: 37175732 Free PMC article. Review.
-
GelMA hydrogels reinforced by PCL@GelMA nanofibers and bioactive glass induce bone regeneration in critical size cranial defects.J Nanobiotechnology. 2024 Nov 11;22(1):696. doi: 10.1186/s12951-024-02980-w. J Nanobiotechnology. 2024. PMID: 39529025 Free PMC article.
-
Bio-integrated scaffold facilitates large bone regeneration dominated by endochondral ossification.Bioact Mater. 2024 Feb 2;35:208-227. doi: 10.1016/j.bioactmat.2024.01.019. eCollection 2024 May. Bioact Mater. 2024. PMID: 38327823 Free PMC article.
References
-
- Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. - PubMed
-
- Burr D.B., Gallant M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012;8:665–673. - PubMed
-
- Gómez-Barrena E., Rosset P., Lozano D., Stanovici J., Ermthaller C., Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

