Lipid-Dependent Titration of Glutamic Acid at a Bilayer Membrane Interface
- PMID: 33817510
- PMCID: PMC8015139
- DOI: 10.1021/acsomega.1c00276
Lipid-Dependent Titration of Glutamic Acid at a Bilayer Membrane Interface
Abstract
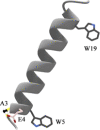
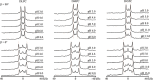
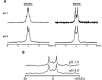
The ionization properties of protein side chains in lipid-bilayer membranes will differ from the canonical values of side chains exposed to an aqueous solution. While the propensities of positively charged side chains of His, Lys, and Arg to release a proton in lipid membranes have been rather well characterized, the propensity for a negatively charged Glu side chain to receive a proton and achieve the neutral state in a bilayer membrane has been less well characterized. Indeed, the ionization of the glutamic acid side chain has been predicted to depend on its depth of burial in a lipid membrane but has been difficult to verify experimentally. To address the issue, we incorporated an interfacial Glu residue at position 4 of a distinct 23-residue transmembrane helix and used 2H NMR to examine the helix properties as a function of pH. We observe that the helix tilt and azimuthal rotation vary little with pH, but the extent of helix unraveling near residues 3 and 4 changes as the Glu residue E4 titrates. Remarkably, the 2H quadrupolar splitting for the side chain of alanine A3 responds to pH with an apparent pK a of 4.8 in 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) and 6.3 in 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC), but is unchanged up to pH 8.0 in 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) in the presence of residue E4. With bilayers composed of alkali-stable ether-linked lipids, the side chain of A3 responds to pH with an apparent pK a of 11.0 in the ether analogue of DOPC. These results suggest that the depth dependence of Glu ionization in lipid-bilayer membranes may be steeper than previously predicted or envisioned.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Influence of Lipid Saturation, Hydrophobic Length and Cholesterol on Double-Arginine-Containing Helical Peptides in Bilayer Membranes.Chembiochem. 2019 Nov 4;20(21):2784-2792. doi: 10.1002/cbic.201900282. Epub 2019 Sep 18. Chembiochem. 2019. PMID: 31150136 Free PMC article.
-
Ionization Properties of Histidine Residues in the Lipid Bilayer Membrane Environment.J Biol Chem. 2016 Sep 2;291(36):19146-56. doi: 10.1074/jbc.M116.738583. Epub 2016 Jul 20. J Biol Chem. 2016. PMID: 27440045 Free PMC article.
-
Charged or aromatic anchor residue dependence of transmembrane peptide tilt.J Biol Chem. 2010 Oct 8;285(41):31723-30. doi: 10.1074/jbc.M110.152470. Epub 2010 Jul 28. J Biol Chem. 2010. PMID: 20667827 Free PMC article.
-
GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery.Adv Drug Deliv Rev. 2004 Apr 23;56(7):967-85. doi: 10.1016/j.addr.2003.10.041. Adv Drug Deliv Rev. 2004. PMID: 15066755 Review.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Surface Properties of Synaptosomes in the Presence of L-Glutamic and Kainic Acids: In Vitro Alteration of the ATPase and Acetylcholinesterase Activities.Membranes (Basel). 2021 Dec 17;11(12):987. doi: 10.3390/membranes11120987. Membranes (Basel). 2021. PMID: 34940488 Free PMC article.
-
Illuminating Disorder Induced by Glu in a Stable Arg-Anchored Transmembrane Helix.ACS Omega. 2021 Jul 26;6(31):20611-20618. doi: 10.1021/acsomega.1c02800. eCollection 2021 Aug 10. ACS Omega. 2021. PMID: 34396006 Free PMC article.
References
-
- Kuhlman B.; Luisi D. L.; Young P.; Raleigh D. P. pK(a) Values and the pH Dependent Stability of the N-terminal Domain of L9 as Probes of Electrostatic Interactions in the Denatured State. Differentiation between Local and Nonlocal Interactions. Biochemistry 1999, 38, 4896–4903. 10.1021/bi982931h. - DOI - PubMed
-
- Caputo G. A.; London E. Position and Ionization State of Asp in the Core of Membrane-Inserted Alpha Helices Control Both the Equilibrium between Transmembrane and Nontransmembrane Helix Topography and Transmembrane Helix Positioning. Biochemistry 2004, 43, 8794–8806. 10.1021/bi049696p. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

