Robustness to High Temperatures of Al2O3-Coated CsPbBr3 Nanocrystal Thin Films with High-Photoluminescence Quantum Yield for Light Emission
- PMID: 33817562
- PMCID: PMC8009476
- DOI: 10.1021/acsanm.0c01525
Robustness to High Temperatures of Al2O3-Coated CsPbBr3 Nanocrystal Thin Films with High-Photoluminescence Quantum Yield for Light Emission
Abstract
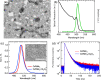
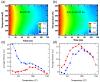
Lead-halide perovskite nanocrystals are a promising material in optical devices due to their high photoluminescence (PL) quantum yield, excellent color purity, and low stimulated emission threshold. However, one problem is the stability of the nanocrystal films under different environmental conditions and under high temperatures. The latter is particularly relevant for device fabrication if further processes that require elevated temperatures are needed after the deposition of the nanocrystal film. In this work, we study the impact of a thin oxide layer of Al2O3 on the light emission properties of thin nanocrystal films. We find that nanocrystals passivated with quaternary ammonium bromide ligands maintain their advantageous optical properties in alumina-coated films and do not suffer from degradation at temperatures up to 100 °C. This is manifested by conservation of the PL peak position and line width, PL decay dynamics, and low threshold for amplified spontaneous emission. The PL remains stable for up to 100 h at a temperature of 80 °C, and the ASE intensity decreases by less than 30% under constant pumping at high fluence for 1 h. Our approach outlines that the combination of tailored surface chemistry with additional protective coating of the nanocrystal film is a feasible approach to obtain stable emission at elevated temperatures and under extended operational time scales.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
