Borrelia burgdorferi sensu lato seroconversion after intravenous immunoglobulin treatment: A cohort study
- PMID: 33817927
- PMCID: PMC8251852
- DOI: 10.1111/ene.14853
Borrelia burgdorferi sensu lato seroconversion after intravenous immunoglobulin treatment: A cohort study
Abstract
Objective: Intravenous immunoglobulin (IVIg) consists of pooled donor immunoglobulins (IgG), possibly including anti-Borrelia burgdorferi (Bbsl) antibodies. Apparent IVIg-related Bbsl seroconversion could lead to incorrect diagnosis of Lyme borreliosis. This cohort study was designed to determine how often IVIg treatment leads to apparent Bbsl seroconversion and whether antibodies disappear post-treatment.
Methods: Sera from chronic inflammatory demyelinating polyneuropathy (CIDP) and myositis patients were analyzed, drawn pre-treatment and 6-12 weeks after the start of IVIg. In patients with apparent seroconversion, follow-up samples after treatment withdrawal were analyzed, if available. Patients treated with corticosteroids were included as controls. A two-tier protocol was used for serological testing consisting of the C6 Lyme ELISA (Oxford Immunotec) and confirmation by immunoglobulin M (IgM) and immunoglobulin G (IgG) immunoblot (Mikrogen® ).
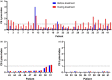
Results: We included 61 patients: 51 patients were treated with IVIg and 10 with dexamethasone. Of the patients treated with IVIg, 42 had CIDP (82%) and were treated with Nanogam® (Sanquin Plasma Products). Nine patients had myositis (18%) and were treated with Privigen® (CSL Behring). Anti-Bbsl IgG seroprevalence pre-treatment was 3% (2/61). Apparent seroconversion during IVIg treatment occurred in 39% (20/51) of patients, all treated with Nanogam. Post-treatment seroreversion occurred in 92% (12/13) of patients with available follow-up samples; in 78% (7/9) seroreversion was observed within 3 months.
Conclusions: Transient presence of anti-Bbsl IgG antibodies after IVIg is regularly observed. This effect appears to be dependent on the IVIg brand, probably reflecting variation in Bbsl exposure of plasma donors. Lyme borreliosis serological testing during, and weeks to months after, IVIg is therefore of limited utility.
Keywords: Borrelia burgdorferi; CIDP; IVIg; apparent seroconversion; intravenous immunoglobulins; myositis.
© 2021 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
I. M. Lucke has nothing to disclose. A. Vrijlandt has nothing to disclose. J. Lim reports financial support from Sanquin for attending a conference, not related to the submitted work. A. J. van der Kooi reports grants from CSL Behring, outside the submitted work. I. N. van Schaik reports grants from Prinses Beatrix Spierfonds, grants from the Netherlands Organization for Scientific Research, and another from CSL Behring, all outside the submitted work. H. Zaaijer has nothing to disclose. J. W. Hovius reports grants from Bio‐Rad Laboratories, grants from the Netherlands Organization for Health Research and Development, and grants from the European Regional Development Fund (INTERREG), outside the submitted work. F. Eftimov reports grants from Prinses Beatrix Spierfonds, the Netherlands Organization for Health Research and Development, a consulting fee from CSL Behring and UCB Pharma that was paid to the author’s institution outside the submitted work, grants from CSL Behring, Kedrion, Terumo BCT, Grifols and Takeda Pharmaceutical Company, outside the submitted work. Grants were paid to the institution and are used for investigator‐initiated studies within INCbase, an international CIDP registry.
Figures
References
-
- Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;1:CD001797. - PubMed
-
- Zóka A, Bekő G. Potential impact of IVIG treatment on Lyme serology. Diagn Microbiol Infect Dis. 2020;96(4):114986. - PubMed
-
- Van den Bergh PYK, Hadden RDM, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society ‐ first revision. Eur J Neurol. 2010;17:356‐363. - PubMed
-
- Bunschoten C, Eftimov F, van der Pol WL, et al. International chronic inflammatory demyelinating polyneuropathy outcome study (ICOS): protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome. J Peripher Nerv Syst. 2019;24(1):34‐38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

