Reduced cellular glucose transport confers natural protection against dextrose-induced superoxide generation and endoplasmic reticulum stress in domestic hen
- PMID: 33818012
- PMCID: PMC8020048
- DOI: 10.14814/phy2.14816
Reduced cellular glucose transport confers natural protection against dextrose-induced superoxide generation and endoplasmic reticulum stress in domestic hen
Abstract
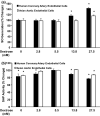
Normal blood glucose levels in avian species are two to fourfold higher than that in humans and the higher blood glucose levels in birds do not cause adverse effects. Endothelial cells isolated from the aorta of the domestic hen (Gallus gallus domesticus) and chicken aortic smooth muscle cells (CAOSMC) were compared to human coronary artery endothelial cells (HCAEC) and human primary aortic smooth muscle cells (HASMC). Superoxide (SO) generation was measured using a superoxide-reactive probe. ER stress was measured using the placental alkaline phosphatase assay (ES-TRAP). Glucose transport kinetics were determined using the 3 H-2-deoxyglucose tracer. Dextrose-induced SO generation and ER stress were significantly blunted in avian endothelial cells compared to human cells. The Vmax of glucose uptake (in nmoles/mg protein/min) in avian endothelial cells (0.0018 ± 0.0001) and smooth muscle cells (0.0015 ± 0.0007) was approximately 18-25 fold lower compared to the Vmax in HCAEC (0.033 ± 0.0025) and HASMC (0.038 ± 0.004) (all p < 0.0001). The Michaelis-Menten constant (Km) of transport was also significantly different (p < 0.0001) in avian species. The relative resistance of avian cells to dextrose-induced oxidative stress and ER stress is mostly the result of reduced cellular dextrose transport.
Keywords: chicken aortic endothelial cells; endoplasmic reticulum stress; glucose transport; human coronary artery endothelial cell; oxidative stress.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Braun, E. J. , & Sweazea, K. L. (2008). Glucose regulation in birds. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 151, 1–9. - PubMed
-
- Campen, H. V. , & Davis, M. R. (1993). Isolation and characterization of chicken aortic endothelial cells. Journal of Tissue Culture Methods, 15, 171–175.
-
- Hayflick, L. (1965). Tissue cultures and mycoplasmas. Texas Reports on Biology and Medicine, 23, 285–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

