Clinical Outcomes Following Letrozole Treatment according to Estrogen Receptor Expression in Postmenopausal Women: LETTER Study (KBCSG-006)
- PMID: 33818022
- PMCID: PMC8090806
- DOI: 10.4048/jbc.2021.24.e17
Clinical Outcomes Following Letrozole Treatment according to Estrogen Receptor Expression in Postmenopausal Women: LETTER Study (KBCSG-006)
Abstract
Purpose: In this trial, we investigated the efficacy and safety of adjuvant letrozole for hormone receptor (HR)-positive breast cancer. Here, we report the clinical outcome in postmenopausal women with HR-positive breast cancer treated with adjuvant letrozole according to estrogen receptor (ER) expression levels.
Methods: In this multi-institutional, open-label, observational study, postmenopausal patients with HR-positive breast cancer received adjuvant letrozole (2.5 mg/daily) for 5 years unless they experienced disease progression or unacceptable toxicity or withdrew their consent. The patients were stratified into the following 3 groups according to ER expression levels using a modified Allred score (AS): low, intermediate, and high (AS 3-4, 5-6, and 7-8, respectively). ER expression was centrally reviewed. The primary objective was the 5-year disease-free survival (DFS) rate.
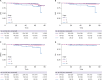
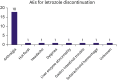
Results: Between April 25, 2010, and February 5, 2014, 440 patients were enrolled. With a median follow-up of 62.0 months, the 5-year DFS rate in all patients was 94.2% (95% confidence interval [CI], 91.8-96.6). The 5-year DFS and recurrence-free survival (RFS) rates did not differ according to ER expression; the 5-year DFS rates were 94.3% and 94.1%in the low-to-intermediate and high expression groups, respectively (p = 0.6), and the corresponding 5-year RFS rates were 95.7% and 95.4%, respectively (p = 0.7). Furthermore, 25 patients discontinued letrozole because of drug toxicity.
Conclusion: Treatment with adjuvant letrozole showed very favorable treatment outcomes and good tolerability among Korean postmenopausal women with ER-positive breast cancer, independent of ER expression.
Trial registration: ClinicalTrials.gov Identifier: NCT01069211.
Keywords: Breast neoplasms; Letrozole; Postmenopause; Receptors, estrogen.
© 2021 Korean Breast Cancer Society.
Conflict of interest statement
This study was funded by Novartis Korea. The funders provided the study drug (letrozole) and collaborated with the authors on the study design and data collection methods.
Figures

References
-
- Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2:1102–1109. - PubMed
-
- Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–1557. - PubMed
-
- Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37:423–438. - PubMed
-
- Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

