Lipophilicity of Cationic Ligands Promotes Irreversible Adsorption of Nanoparticles to Lipid Bilayers
- PMID: 33818061
- PMCID: PMC9153949
- DOI: 10.1021/acsnano.0c09732
Lipophilicity of Cationic Ligands Promotes Irreversible Adsorption of Nanoparticles to Lipid Bilayers
Abstract
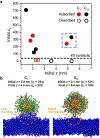
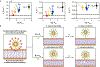
A mechanistic understanding of the influence of the surface properties of engineered nanomaterials on their interactions with cells is essential for designing materials for applications such as bioimaging and drug delivery as well as for assessing nanomaterial safety. Ligand-coated gold nanoparticles have been widely investigated because their highly tunable surface properties enable investigations into the effect of ligand functionalization on interactions with biological systems. Lipophilic ligands have been linked to adverse biological outcomes through membrane disruption, but the relationship between ligand lipophilicity and membrane interactions is not well understood. Here, we use a library of cationic ligands coated on 2 nm gold nanoparticles to probe the impact of ligand end group lipophilicity on interactions with supported phosphatidylcholine lipid bilayers as a model for cytoplasmic membranes. Nanoparticle adsorption to and desorption from the model membranes were investigated by quartz crystal microbalance with dissipation monitoring. We find that nanoparticle adsorption to model membranes increases with ligand lipophilicity. The effects of ligand structure on gold nanoparticle attachment were further analyzed using atomistic molecular dynamics simulations, which showed that the increase in ligand lipophilicity promotes ligand intercalation into the lipid bilayer. Together, the experimental and simulation results could be described by a two-state model that accounts for the initial attachment and subsequent conversion to a quasi-irreversibly bound state. We find that only nanoparticles coated with the most lipophilic ligands in our nanoparticle library undergo conversion to the quasi-irreversible state. We propose that the initial attachment is governed by interaction between the ligands and phospholipid tail groups, whereas conversion into the quasi-irreversibly bound state reflects ligand intercalation between phospholipid tail groups and eventual lipid extraction from the bilayer. The systematic variation of ligand lipophilicity enabled us to demonstrate that the lipophilicity of cationic ligands correlates with nanoparticle-bilayer adsorption and suggested that changing the nonpolar ligand R group promotes a mechanism of ligand intercalation into the bilayer associated with irreversible adsorption.
Keywords: classical molecular dynamics simulations; ligand-coated gold nanoparticles; nanobio interface; quartz crystal microbalance with dissipation monitoring; structure−property relationship; supported lipid bilayers; umbrella sampling.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Johnston LJ; Gonzalez-Rojano N; Wilkinson KJ; Xing B Key Challenges for Evaluation of the Safety of Engineered Nanomaterials. NanoImpact 2020, 18, 100219. 10.1016/j.impact.2020.100219. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

