Astrocyte senescence and SASP in neurodegeneration: tau joins the loop
- PMID: 33818291
- PMCID: PMC8098067
- DOI: 10.1080/15384101.2021.1909260
Astrocyte senescence and SASP in neurodegeneration: tau joins the loop
Abstract
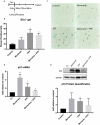
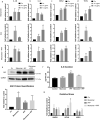
Tau accumulation is a core component of Alzheimer's disease and other neurodegenerative tauopathies. While tau's impact on neurons is a major area of research, the effect of extracellular tau on astrocytes is largely unknown. This article summarizes our recent studies showing that astrocyte senescence plays a critical role in neurodegenerative diseases and integrates extracellular tau into the regulatory loop of senescent astrocyte-mediated neurotoxicity. Human astrocytes in vitro undergoing senescence were shown to acquire the inflammatory senescence-associated secretory phenotype (SASP) and toxicity to neurons, which may recapitulate aging- and disease-associated neurodegeneration. Here, we show that human astrocytes exposed to extracellular tau in vitro also undergo cellular senescence and acquire a neurotoxic SASP (e.g. IL-6 secretion), with oxidative stress response (indicated by upregulated NRF2 target genes) and a possible activation of inflammasome (indicated by upregulated ASC and IL-1β). These findings suggest that senescent astrocytes induced by various conditions and insults, including tau exposure, may represent a therapeutic target to inhibit or delay the progression of neurodegenerative diseases. We also discuss the pathological activity of extracellular tau in microglia and astrocytes, the disease relevance and diversity of tau forms, therapeutics targeting senescence in neurodegeneration, and the roles of p53 and its isoforms in astrocyte-mediated neurotoxicity and neuroprotection.
Keywords: Alzheimer’s disease; SASP; astrocyte senescence; neurodegeneration; p53 isoforms; tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
SerpinA3N Regulates the Secretory Phenotype of Mouse Senescent Astrocytes Contributing to Neurodegeneration.J Gerontol A Biol Sci Med Sci. 2024 Apr 1;79(4):glad278. doi: 10.1093/gerona/glad278. J Gerontol A Biol Sci Med Sci. 2024. PMID: 38175667
-
p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration.Cell Death Differ. 2016 Sep 1;23(9):1515-28. doi: 10.1038/cdd.2016.37. Epub 2016 Apr 22. Cell Death Differ. 2016. PMID: 27104929 Free PMC article.
-
Cellular Senescence as the Key Intermediate in Tau-Mediated Neurodegeneration.Rejuvenation Res. 2018 Dec;21(6):572-579. doi: 10.1089/rej.2018.2155. Rejuvenation Res. 2018. PMID: 30489222
-
Astrocyte senescence: Evidence and significance.Aging Cell. 2019 Jun;18(3):e12937. doi: 10.1111/acel.12937. Epub 2019 Feb 27. Aging Cell. 2019. PMID: 30815970 Free PMC article. Review.
-
The Role of Glial Cell Senescence in Alzheimer's Disease.J Neurochem. 2025 Mar;169(3):e70051. doi: 10.1111/jnc.70051. J Neurochem. 2025. PMID: 40130281 Free PMC article. Review.
Cited by
-
Gut microbiota-astrocyte axis: new insights into age-related cognitive decline.Neural Regen Res. 2025 Apr 1;20(4):990-1008. doi: 10.4103/NRR.NRR-D-23-01776. Epub 2024 Apr 16. Neural Regen Res. 2025. PMID: 38989933 Free PMC article.
-
The interaction between ageing and Alzheimer's disease: insights from the hallmarks of ageing.Transl Neurodegener. 2024 Jan 23;13(1):7. doi: 10.1186/s40035-024-00397-x. Transl Neurodegener. 2024. PMID: 38254235 Free PMC article. Review.
-
Doxorubicin Induces a Senescent Phenotype in Murine and Human Astrocytes.J Neurochem. 2025 Aug;169(8):e70177. doi: 10.1111/jnc.70177. J Neurochem. 2025. PMID: 40762103 Free PMC article.
-
The relationships between neuroglial alterations and neuronal changes in Alzheimer's disease, and the related controversies I: Gliopathogenesis and glioprotection.J Cent Nerv Syst Dis. 2022 Oct 9;14:11795735221128703. doi: 10.1177/11795735221128703. eCollection 2022. J Cent Nerv Syst Dis. 2022. PMID: 36238130 Free PMC article. Review.
-
Intraneuronal tau aggregation induces the integrated stress response in astrocytes.J Mol Cell Biol. 2023 Mar 29;14(10):mjac071. doi: 10.1093/jmcb/mjac071. J Mol Cell Biol. 2023. PMID: 36520068 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
