TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL
- PMID: 33818299
- PMCID: PMC8023237
- DOI: 10.1080/19420862.2021.1890411
TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL
Abstract
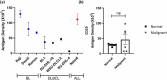
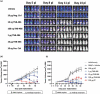
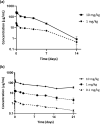
The therapeutic potential of targeting CD19 in B cell malignancies has garnered attention in the past decade, resulting in the introduction of novel immunotherapy agents. Encouraging clinical data have been reported for T cell-based targeting agents, such as anti-CD19/CD3 bispecific T-cell engager blinatumomab and chimeric antigen receptor (CAR)-T therapies, for acute lymphoblastic leukemia and B cell non-Hodgkin lymphoma (B-NHL). However, clinical use of both blinatumomab and CAR-T therapies has been limited due to unfavorable pharmacokinetics (PK), significant toxicity associated with cytokine release syndrome and neurotoxicity, and manufacturing challenges. We present here a fully human CD19xCD3 bispecific antibody (TNB-486) for the treatment of B-NHL that could address the limitations of the current approved treatments. In the presence of CD19+ target cells and T cells, TNB-486 induces tumor cell lysis with minimal cytokine release, when compared to a positive control. In vivo, TNB-486 clears CD19+ tumor cells in immunocompromised mice in the presence of human peripheral blood mononuclear cells in multiple models. Additionally, the PK of TNB-486 in mice or cynomolgus monkeys is similar to conventional antibodies. This new T cell engaging bispecific antibody targeting CD19 represents a novel therapeutic that induces potent T cell-mediated tumor-cell cytotoxicity uncoupled from high levels of cytokine release, making it an attractive candidate for B-NHL therapy.
Keywords: Bispecific antibody; CD19XCD3; T-cell engager; TNB-486; cytokine release syndrome; low cytokine release.
Conflict of interest statement
All authors are current or former employees of Teneobio, Inc. with equity interests.
Figures




Similar articles
-
A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells.MAbs. 2015;7(3):584-604. doi: 10.1080/19420862.2015.1029216. MAbs. 2015. PMID: 25875246 Free PMC article.
-
Redirection of CD4+ and CD8+ T lymphocytes via an anti-CD3 × anti-CD19 bi-specific antibody combined with cytosine arabinoside and the efficient lysis of patient-derived B-ALL cells.J Hematol Oncol. 2015 Oct 6;8:108. doi: 10.1186/s13045-015-0205-6. J Hematol Oncol. 2015. PMID: 26444983 Free PMC article.
-
CD47/SIRPα blocking enhances CD19/CD3-bispecific T cell engager antibody-mediated lysis of B cell malignancies.Biochem Biophys Res Commun. 2019 Feb 12;509(3):739-745. doi: 10.1016/j.bbrc.2018.12.175. Epub 2019 Jan 3. Biochem Biophys Res Commun. 2019. PMID: 30611570
-
Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy.Leuk Lymphoma. 2016 May;57(5):1021-32. doi: 10.3109/10428194.2016.1161185. Epub 2016 Apr 6. Leuk Lymphoma. 2016. PMID: 27050240 Review.
-
Concepts in immuno-oncology: tackling B cell malignancies with CD19-directed bispecific T cell engager therapies.Ann Hematol. 2020 Oct;99(10):2215-2229. doi: 10.1007/s00277-020-04221-0. Epub 2020 Aug 27. Ann Hematol. 2020. PMID: 32856140 Free PMC article. Review.
Cited by
-
Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release.J Immunother Cancer. 2021 Jun;9(6):e002488. doi: 10.1136/jitc-2021-002488. J Immunother Cancer. 2021. PMID: 34088740 Free PMC article.
-
T-cell redirecting therapies for B-cell non-Hodgkin lymphoma: recent progress and future directions.Front Oncol. 2023 Jul 3;13:1168622. doi: 10.3389/fonc.2023.1168622. eCollection 2023. Front Oncol. 2023. PMID: 37465110 Free PMC article. Review.
-
Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies.J Hematol Oncol. 2023 Jul 27;16(1):83. doi: 10.1186/s13045-023-01482-w. J Hematol Oncol. 2023. PMID: 37501154 Free PMC article. Review.
-
Promising Immunotherapeutic Modalities for B-Cell Lymphoproliferative Disorders.Int J Mol Sci. 2021 Oct 25;22(21):11470. doi: 10.3390/ijms222111470. Int J Mol Sci. 2021. PMID: 34768899 Free PMC article. Review.
-
CLN-978, a novel half-life extended CD19/CD3/HSA-specific T cell-engaging antibody construct with potent activity against B-cell malignancies with low CD19 expression.J Immunother Cancer. 2023 Aug;11(8):e007398. doi: 10.1136/jitc-2023-007398. J Immunother Cancer. 2023. PMID: 37586770 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
