Rapid chromatographic immunoassay-based evaluation of COVID-19: A cross-sectional, diagnostic test accuracy study & its implications for COVID-19 management in India
- PMID: 33818469
- PMCID: PMC8184078
- DOI: 10.4103/ijmr.IJMR_3305_20
Rapid chromatographic immunoassay-based evaluation of COVID-19: A cross-sectional, diagnostic test accuracy study & its implications for COVID-19 management in India
Abstract
Background & objectives: Coronavirus disease 2019 (COVID-19) has so far affected over 41 million people globally. The limited supply of real-time reverse transcription-polymerase chain reaction (rRT-PCR) kits and reagents has made meeting the rising demand for increased testing incompetent, worldwide. A highly sensitive and specific antigen-based rapid diagnostic test (RDT) is the need of the hour. The objective of this study was to evaluate the performance of a rapid chromatographic immunoassay-based test (index test) compared with a clinical reference standard (rRT-PCR).
Methods: A cross-sectional, single-blinded study was conducted at a tertiary care teaching hospital in north India. Paired samples were taken for RDT and rRT-PCR (reference standard) from consecutive participants screened for COVID-19 to calculate the sensitivity and specificity of the RDT. Further subgroup analysis was done based on the duration of illness and cycle threshold values. Cohen's kappa coefficient was used to measure the level of agreement between the two tests.
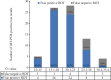
Results: Of the 330 participants, 77 were rRT-PCR positive for SARS-CoV-2. Sixty four of these patients also tested positive for SARS-CoV-2 by RDT. The overall sensitivity and specificity were 81.8 and 99.6 per cent, respectively. The sensitivity of RDT was higher (85.9%) in participants with a duration of illness ≤5 days.
Interpretation & conclusions: With an excellent specificity and moderate sensitivity, this RDT may be used to rule in COVID-19 in patients with a duration of illness ≤5 days. Large-scale testing based on this RDT across the country would result in quick detection, isolation and treatment of COVID-19 patients.
Keywords: Antigen test; COVID-19; SARS-CoV-2; point-of-care test.
Conflict of interest statement
None
Figures

References
-
- World Health Organization. Coronavirus disease 2019: Events as they happen. [accessed on June 29, 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a... .
-
- World Health Organization. Advice on the use of pointof- care immunodiagnostic tests for COVID-19: Scientific brief, 8 April 2020. [accessed on October 22, 2020]. Available from: https://apps.who.int/iris/handle/10665/331713 .
-
- Government of India. India Fights Corona COVID-19. [accessed on July 14, 2020]. Available from: https://www.mygov.in/covid-19/
-
- Centers for Disease Control and Prevention. CDC 2019-novel coronavirus (2019- nCoV) real-time RT-PCR diagnostic panel. Atlanta: CDC; 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
