Low-dose metformin treatment in the subacute phase improves the locomotor function of a mouse model of spinal cord injury
- PMID: 33818507
- PMCID: PMC8354108
- DOI: 10.4103/1673-5374.310695
Low-dose metformin treatment in the subacute phase improves the locomotor function of a mouse model of spinal cord injury
Abstract
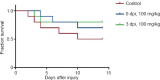
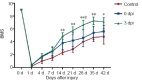
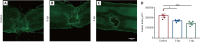
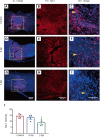
Metformin, a first-line drug for type-2 diabetes, has been shown to improve locomotor recovery after spinal cord injury. However, there are studies reporting no beneficial effect. Recently, we found that high dose of metformin (200 mg/kg, intraperitoneal) and acute phase administration (immediately after injury) led to increased mortality and limited locomotor function recovery. Consequently, we used a lower dose (100 mg/kg, i.p.) metformin in mice, and compared the effect of immediate administration after spinal cord injury (acute phase) with that of administration at 3 days post-injury (subacute phase). Our data showed that metformin treatment starting at the subacute phase significantly improved mouse locomotor function evaluated by Basso Mouse Scale (BMS) scoring. Immunohistochemical studies also revealed significant inhibitions of microglia/macrophage activation and astrogliosis at the lesion site. Furthermore, metformin treatment at the subacute phase reduced neutrophil infiltration. These changes were in parallel with the increased survival rate of spinal neurons in animals treated with metformin. These findings suggest that low-dose metformin treatment for subacute spinal cord injury can effectively improve the functional recovery possibly through anti-inflammation and neuroprotection. This study was approved by the Institute Animal Care and Use Committee at the University of Texas Medical Branch (approval No. 1008041C) in 2010.
Keywords: inflammation; locomotor function; metformin; microglia; mortality; neuroprotection; spinal cord injury; subacute administration.
Conflict of interest statement
None
Figures



Similar articles
-
Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats.J Neurochem. 2016 May;137(4):604-17. doi: 10.1111/jnc.13610. Epub 2016 Apr 12. J Neurochem. 2016. PMID: 26998684 Free PMC article.
-
An Agonist of the Protective Factor SIRT1 Improves Functional Recovery and Promotes Neuronal Survival by Attenuating Inflammation after Spinal Cord Injury.J Neurosci. 2017 Mar 15;37(11):2916-2930. doi: 10.1523/JNEUROSCI.3046-16.2017. Epub 2017 Feb 13. J Neurosci. 2017. PMID: 28193684 Free PMC article.
-
Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury.J Neurosurg. 2014 Dec;121(6):1514-25. doi: 10.3171/2014.8.JNS132562. Epub 2014 Oct 3. J Neurosurg. 2014. PMID: 25280090
-
Osteopontin-deficient mice exhibit less inflammation, greater tissue damage, and impaired locomotor recovery from spinal cord injury compared with wild-type controls.J Neurosci. 2007 Mar 28;27(13):3603-11. doi: 10.1523/JNEUROSCI.4805-06.2007. J Neurosci. 2007. PMID: 17392476 Free PMC article.
-
Give progesterone a chance.Neural Regen Res. 2014 Aug 1;9(15):1422-4. doi: 10.4103/1673-5374.139456. Neural Regen Res. 2014. PMID: 25317151 Free PMC article. Review.
Cited by
-
Temporal changes of spinal microglia in murine models of neuropathic pain: a scoping review.Front Immunol. 2024 Dec 6;15:1460072. doi: 10.3389/fimmu.2024.1460072. eCollection 2024. Front Immunol. 2024. PMID: 39735541 Free PMC article.
-
Metformin promotes angiogenesis and functional recovery in aged mice after spinal cord injury by adenosine monophosphate-activated protein kinase/endothelial nitric oxide synthase pathway.Neural Regen Res. 2023 Jul;18(7):1553-1562. doi: 10.4103/1673-5374.360245. Neural Regen Res. 2023. PMID: 36571362 Free PMC article.
-
Metformin's therapeutic potential in spinal cord injury: a systematic review and meta-analysis on locomotor recovery, neuropathic pain alleviation, and modulation of secondary injury mechanisms.Acta Neurochir (Wien). 2025 Mar 24;167(1):87. doi: 10.1007/s00701-025-06487-7. Acta Neurochir (Wien). 2025. PMID: 40126598 Free PMC article.
-
3,4-Dimethoxychalcone, a caloric restriction mimetic, enhances TFEB-mediated autophagy and alleviates pyroptosis and necroptosis after spinal cord injury.Theranostics. 2023 Jan 1;13(2):810-832. doi: 10.7150/thno.78370. eCollection 2023. Theranostics. 2023. PMID: 36632211 Free PMC article.
-
Network pharmacology integrated with experimental validation to explore the therapeutic role and potential mechanism of Epimedium for spinal cord injury.Front Mol Neurosci. 2023 Jan 30;16:1074703. doi: 10.3389/fnmol.2023.1074703. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36793356 Free PMC article.
References
-
- Afshari K, Dehdashtian A, Haddadi NS, Haj-Mirzaian A, Iranmehr A, Ebrahimi MA, Tavangar SM, Faghir-Ghanesefat H, Mohammadi F, Rahimi N, Javidan AN, Dehpour AR. Anti-inflammatory effects of metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord. 2018;56:1032–1041. - PubMed
-
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687–700. - PubMed
-
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. - PMC - PubMed
-
- Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. - PubMed
-
- Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10:580–583. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

