Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial
- PMID: 33818600
- PMCID: PMC8022268
- DOI: 10.1001/jamaneurol.2021.0538
Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial
Abstract
Importance: Many patients with diabetic peripheral neuropathy experience chronic pain and inadequate relief despite best available medical treatments.
Objective: To determine whether 10-kHz spinal cord stimulation (SCS) improves outcomes for patients with refractory painful diabetic neuropathy (PDN).
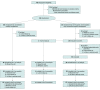
Design, setting, and participants: The prospective, multicenter, open-label SENZA-PDN randomized clinical trial compared conventional medical management (CMM) with 10-kHz SCS plus CMM. Participants with PDN for 1 year or more refractory to gabapentinoids and at least 1 other analgesic class, lower limb pain intensity of 5 cm or more on a 10-cm visual analogue scale (VAS), body mass index (calculated as weight in kilograms divided by height in meters squared) of 45 or less, hemoglobin A1c (HbA1c) of 10% or less, daily morphine equivalents of 120 mg or less, and medically appropriate for the procedure were recruited from clinic patient populations and digital advertising. Participants were enrolled from multiple sites across the US, including academic centers and community pain clinics, between August 2017 and August 2019 with 6-month follow-up and optional crossover at 6 months. Screening 430 patients resulted in 214 who were excluded or declined participation and 216 who were randomized. At 6-month follow-up, 187 patients were evaluated.
Interventions: Implanted medical device delivering 10-kHz SCS.
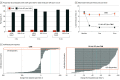
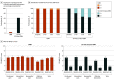
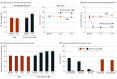
Main outcomes and measures: The prespecified primary end point was percentage of participants with 50% pain relief or more on VAS without worsening of baseline neurological deficits at 3 months. Secondary end points were tested hierarchically, as prespecified in the analysis plan. Measures included pain VAS, neurological examination, health-related quality of life (EuroQol Five-Dimension questionnaire), and HbA1c over 6 months.
Results: Of 216 randomized patients, 136 (63.0%) were male, and the mean (SD) age was 60.8 (10.7) years. Additionally, the median (interquartile range) duration of diabetes and peripheral neuropathy were 10.9 (6.3-16.4) years and 5.6 (3.0-10.1) years, respectively. The primary end point assessed in the intention-to-treat population was met by 5 of 94 patients in the CMM group (5%) and 75 of 95 patients in the 10-kHz SCS plus CMM group (79%; difference, 73.6%; 95% CI, 64.2-83.0; P < .001). Infections requiring device explant occurred in 2 patients in the 10-kHz SCS plus CMM group (2%). For the CMM group, the mean pain VAS score was 7.0 cm (95% CI, 6.7-7.3) at baseline and 6.9 cm (95% CI, 6.5-7.3) at 6 months. For the 10-kHz SCS plus CMM group, the mean pain VAS score was 7.6 cm (95% CI, 7.3-7.9) at baseline and 1.7 cm (95% CI, 1.3-2.1) at 6 months. Investigators observed neurological examination improvements for 3 of 92 patients in the CMM group (3%) and 52 of 84 in the 10-kHz SCS plus CMM group (62%) at 6 months (difference, 58.6%; 95% CI, 47.6-69.6; P < .001).
Conclusions and relevance: Substantial pain relief and improved health-related quality of life sustained over 6 months demonstrates 10-kHz SCS can safely and effectively treat patients with refractory PDN.
Trial registration: ClincalTrials.gov Identifier: NCT03228420.
Conflict of interest statement
Figures
Comment in
-
High-frequency spinal cord stimulation alleviates painful diabetic neuropathy.Nat Rev Neurol. 2021 May;17(5):262. doi: 10.1038/s41582-021-00493-w. Nat Rev Neurol. 2021. PMID: 33846616 No abstract available.
References
-
- World Health Organization . Global report on diabetes, 2016. Accessed November 9, 2018. https://apps.who.int/iris/rest/bitstreams/909883/retrieve
-
- Bril V, England J, Franklin GM, et al. ; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

