Effects of osteogenic ambulatory mechanical stimulation on early stages of BMP-2 mediated bone repair
- PMID: 33820456
- PMCID: PMC8490484
- DOI: 10.1080/03008207.2021.1897582
Effects of osteogenic ambulatory mechanical stimulation on early stages of BMP-2 mediated bone repair
Abstract
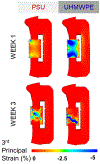
Purpose: Mechanical loading of bone defects through rehabilitation is a promising approach to stimulate repair and reduce nonunion risk; however, little is known about how therapeutic mechanical stimuli modulate early-stage repair before mineralized bone formation. The objective of this study was to investigate the early effects of osteogenic loading on cytokine expression and angiogenesis during the first 3 weeks of BMP-2 mediated segmental bone defect repair.Materials and Methods: A rat model of BMP-2 mediated bone defect repair was subjected to an osteogenic mechanical loading protocol using ambulatory rehabilitation and a compliant, load-sharing fixator with an integrated implantable strain sensor. The effect of fixator load-sharing on local tissue strain, angiogenesis, and cytokine expression was evaluated.Results: Using sensor readings for local measurements of boundary conditions, finite element simulations showed strain became amplified in remaining soft tissue regions between 1 and 3 weeks (Week 3: load-sharing: -1.89 ± 0.35% and load-shielded: -1.38 ± 0.35% vs. Week 1: load-sharing: -1.54 ± 0.17%; load-shielded: -0.76 ± 0.06%). Multivariate analysis of cytokine arrays revealed that load-sharing significantly altered expression profiles in the defect tissue at 2 weeks compared to load-shielded defects. Specifically, loading reduced VEGF (p = 0.052) and increased CXCL5 (LIX) levels. Subsequently, vascular volume in loaded defects was reduced relative to load-shielded defects but similar to intact bone at 3 weeks. Endochondral bone repair was also observed histologically in loaded defects at 3 weeks.Conclusions: Together, these results demonstrate that moderate ambulatory strains previously shown to stimulate bone regeneration significantly alter early angiogenic and cytokine signaling and may promote endochondral ossification.
Keywords: Bone repair; angiogenesis word; immune response; mechanobiology; rehabilitation.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
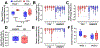
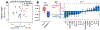
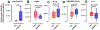

References
-
- Zura R, Xiong Z, Einhorn T, et al. 2016. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 151(11):e162775 [cited 2016 Dec 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/27603155. - PubMed
-
- Sebbag E, Felten R, Sagez F, et al. 2019. The world-wide burden of musculoskeletal diseases: a systematic analysis of the World Health Organization Burden of Diseases Database. Ann. Rheum. Dis. :annrheumdis-2019-215142. - PubMed
-
- Morgan EF, Salisbury Palomares KT, Gleason RE, et al. 2010. Correlations between local strains and tissue phenotypes in an experimental model of skeletal healing. J. Biomech. 43(12):2418–24 [cited 2015 Jan 9] Available from: http://www.jbiomech.com/article/S0021929010002319/fulltext. - PMC - PubMed
-
- Claes LE, Claes LE, Heigele C a, et al. 1998. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 355S:S132–47 Available from: http://www.ncbi.nlm.nih.gov/pubmed/9917634. - PubMed
-
- Klosterhoff BS, Nagaraja S, Dedania JJ, et al. 2017. Material and Mechanobiological Considerations for Bone Regeneration.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
