Rapid visual detection of SARS-CoV-2 by colorimetric loop-mediated isothermal amplification
- PMID: 33820475
- PMCID: PMC8023013
- DOI: 10.2144/btn-2020-0159
Rapid visual detection of SARS-CoV-2 by colorimetric loop-mediated isothermal amplification
Abstract
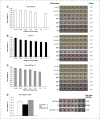
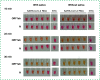
Evaluation of the performance of a new set of primers defined from the ORF1ab sequence, and its combination with a previously published set of primers from the N sequence, to detect SARS-CoV-2 RNA by the loop-mediated isothermal amplification technique is presented. The ORF1ab primer set enables visual detection of SARS-CoV-2 RNA in 16 min. In addition, a simultaneous reaction with both ORF1ab and N primers allows for higher sensitivity of detection, particularly when low numbers of copies are present (250 viral RNA copies). Further, the protocol is able to detect viral RNA in saliva samples. The procedure reported could be easily implemented in the generation of a new and sensitive rapid point-of care device for SARS-CoV-2 RNA visual detection.
Keywords: COVID-19; ORF1ab primers; SARS-CoV-2 detection; nucleocapsid primers; virus LAMP amplification.
Figures

Similar articles
-
Development and comparison of novel multiple cross displacement amplification (MCDA) assays with other nucleic acid amplification methods for SARS-CoV-2 detection.Sci Rep. 2021 Jan 21;11(1):1873. doi: 10.1038/s41598-021-81518-8. Sci Rep. 2021. PMID: 33479389 Free PMC article.
-
Colorimetric Reverse Transcription-Loop-Mediated Isothermal Amplification Assay for Rapid Detection of SARS-CoV-2.Am J Trop Med Hyg. 2021 Jun 15;105(2):375-377. doi: 10.4269/ajtmh.21-0150. Am J Trop Med Hyg. 2021. PMID: 34129521 Free PMC article.
-
Rapid and Extraction-Free Detection of SARS-CoV-2 from Saliva by Colorimetric Reverse-Transcription Loop-Mediated Isothermal Amplification.Clin Chem. 2021 Jan 30;67(2):415-424. doi: 10.1093/clinchem/hvaa267. Clin Chem. 2021. PMID: 33098427 Free PMC article.
-
Loop-mediated isothermal amplification (LAMP): An effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2.Rev Med Virol. 2021 Nov;31(6):e2215. doi: 10.1002/rmv.2215. Epub 2021 Jan 21. Rev Med Virol. 2021. PMID: 33476080 Free PMC article. Review.
-
Recent developments in isothermal amplification technology for rapid detection of SARS-CoV-2.Anal Methods. 2025 Jan 23;17(4):652-664. doi: 10.1039/d4ay01106f. Anal Methods. 2025. PMID: 39679561 Review.
Cited by
-
SARS-CoV-2 Diagnostics Based on Nucleic Acids Amplification: From Fundamental Concepts to Applications and Beyond.Front Cell Infect Microbiol. 2022 Mar 23;12:799678. doi: 10.3389/fcimb.2022.799678. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402302 Free PMC article. Review.
-
Rapid molecular diagnostics of large deletional β0-thalassemia (3.5 kb and 45 kb) using colorimetric LAMP in various thalassemia genotypes.Heliyon. 2021 Nov 12;7(11):e08372. doi: 10.1016/j.heliyon.2021.e08372. eCollection 2021 Nov. Heliyon. 2021. PMID: 34816050 Free PMC article.
-
Developing RT-LAMP assays for rapid diagnosis of SARS-CoV-2 in saliva.EBioMedicine. 2022 Jan;75:103736. doi: 10.1016/j.ebiom.2021.103736. Epub 2021 Dec 16. EBioMedicine. 2022. PMID: 34922321 Free PMC article.
-
Label-free sensing of virus-like particles below the sub-diffraction limit by wide-field photon state parametric imaging of a gold nanodot array.Nanoscale Adv. 2021 Oct 23;3(24):6882-6887. doi: 10.1039/d1na00603g. eCollection 2021 Dec 7. Nanoscale Adv. 2021. PMID: 36132363 Free PMC article.
-
Detection of emerging genotypes in Trichophyton mentagrophytes species complex: A proposal for handling biodiversity in dermatophytes.Front Microbiol. 2022 Aug 23;13:960190. doi: 10.3389/fmicb.2022.960190. eCollection 2022. Front Microbiol. 2022. PMID: 36081804 Free PMC article.
References
-
- Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and clinical characteristics of COVID-19. Arch. Iran Med. 23(4), 268–271 (2020). - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
