Lessons from pathophysiology: Use of individualized combination treatments with immune interventional agents to tackle severe respiratory failure in patients with COVID-19
- PMID: 33820686
- PMCID: PMC7997723
- DOI: 10.1016/j.ejim.2021.03.026
Lessons from pathophysiology: Use of individualized combination treatments with immune interventional agents to tackle severe respiratory failure in patients with COVID-19
Abstract
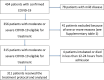
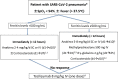
Aims Infection by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) may lead to the development of severe respiratory failure. In hospitalized-patients, prompt interruption of the virus-driven inflammatory process by using combination treatments seems theoretically of outmost importance. Our aim was to investigate the hypothesis of multifaceted management of these patients. Methods A treatment algorithm based on ferritin was applied in 311 patients (67.2% males; median age 63-years; moderate disease, n=101; severe, n=210). Patients with ferritin <500ng/ml received anakinra 2-4mg/kg/day ± corticosteroids (Arm A, n=142) while those with ≥500ng/ml received anakinra 5-8mg/kg/day with corticosteroids and γ-globulins (Arm B, n=169). In case of no improvement a single dose of tocilizumab (8mg/kg; maximum 800mg) was administered with the potential of additional second and/or third pulses. Treatment endpoints were the rate of the development of respiratory failure necessitating intubation and the SARS-CoV-2-related mortality. The proposed algorithm was also validated in matched hospitalized-patients treated with standard-of-care during the same period. Results In overall, intubation and mortality rates were 5.8% and 5.1% (0% in moderate; 8.6% and 7.6% in severe). Low baseline pO2/FiO2 and older age were independent risk factors. Comparators had significantly higher intubation (HR=7.4; 95%CI: 4.1-13.4; p<0.001) and death rates (HR=4.5, 95%CI: 2.1-9.4, p<0.001). Significant adverse events were rare, including severe secondary infections in only 7/311 (2.3%). Conclusions Early administration of personalized combinations of immunomodulatory agents may be life-saving in hospitalized-patients with COVID-19. An immediate intervention (the sooner the better) could be helpful to avoid development of full-blown acute respiratory distress syndrome and improve survival.
Keywords: Anakinra; COVID-19; IL-1; IL-6; SARS-CoV-2; Tocilizumab.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
George N Dalekos is an advisor or lecturer for Ipsen, Pfizer, Genkyotex, Novartis, Sobi, received research grants from Abbvie, Gilead and has served as PI in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics Inc, Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics Inc., Sobi and Intercept Pharmaceuticals. E.J. Giamarellos-Bourboulis has received honoraria from Abbott CH, Angelini Italy, InflaRx GmbH, MSD Greece, XBiotech Inc., and B•R•A•H•M•S GmbH (Thermo Fisher Scientific); independent educational grants from AbbVie Inc, Abbott CH, Astellas Pharma Europe, AxisShield, bioMérieux Inc, Novartis, InflaRx GmbH, and XBiotech Inc; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). The rest authors declare they have no conflict of interest.
Figures



References
-
- Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

