Nitrate-Responsive Suppression of Dimethyl Sulfoxide Respiration in a Facultative Anaerobic Haloarchaeon, Haloferax volcanii
- PMID: 33820797
- PMCID: PMC8316033
- DOI: 10.1128/JB.00655-20
Nitrate-Responsive Suppression of Dimethyl Sulfoxide Respiration in a Facultative Anaerobic Haloarchaeon, Haloferax volcanii
Abstract
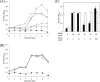
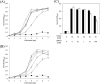
Haloferax volcanii is a facultative anaerobic haloarchaeon that can grow using nitrate or dimethyl sulfoxide (DMSO) as a respiratory substrate under anaerobic conditions. Comparative transcriptome analysis of denitrifying and aerobic cells of H. volcanii indicated extensive changes in gene expression involving the activation of denitrification, suppression of DMSO respiration, and conversion of the heme biosynthetic pathway under denitrifying conditions. The anaerobic growth of H. volcanii by DMSO respiration was inhibited at nitrate concentrations of <1 mM, whereas nitrate-responsive growth inhibition was not observed in the ΔnarO mutant. A reporter assay demonstrated that the transcription of the dms operon was suppressed by nitrate. In contrast, the anaerobic growth of the ΔdmsR mutant by denitrification was little affected by the addition of DMSO. NarO has been identified as an activator of denitrification-related genes in response to anaerobic conditions, and here, we found that NarO is also involved in nitrate-responsive suppression of the dms operon. Nitrate-responsive suppression of DMSO respiration is known in several bacteria such as Escherichia coli and photosynthetic Rhodobacter species. This is the first report to show that a regulatory mechanism that suppresses DMSO respiration in response to nitrate exists not only in bacteria but also in haloarchaea. IMPORTANCE Haloferax volcanii can grow anaerobically by denitrification (nitrate respiration) or DMSO respiration. In facultative anaerobic bacteria that can grow by both nitrate respiration and DMSO respiration, nitrate respiration is preferentially induced when both nitrate and DMSO are available as the respiratory substrates. The results of transcriptome analysis, growth phenotyping, and reporter assays indicated that DMSO respiration is suppressed in response to nitrate in H. volcanii. The haloarchaeon-specific regulator NarO, which activates denitrification under anaerobic conditions, is suggested to be involved in the nitrate-responsive suppression of DMSO respiration.
Keywords: DMSO reductase; DMSO respiration; DmsR; Haloferax volcanii; NarO; denitrification; haloarchaea; nitrate reductase; transcription regulator.
Figures


Similar articles
-
Anaerobic Growth of Haloarchaeon Haloferax volcanii by Denitrification Is Controlled by the Transcription Regulator NarO.J Bacteriol. 2016 Jan 19;198(7):1077-86. doi: 10.1128/JB.00833-15. J Bacteriol. 2016. PMID: 26787768 Free PMC article.
-
Transcriptional regulation of dimethyl sulfoxide respiration in a haloarchaeon, Haloferax volcanii.Extremophiles. 2016 Jan;20(1):27-36. doi: 10.1007/s00792-015-0794-6. Epub 2015 Oct 28. Extremophiles. 2016. PMID: 26507955
-
Genomic analysis of anaerobic respiration in the archaeon Halobacterium sp. strain NRC-1: dimethyl sulfoxide and trimethylamine N-oxide as terminal electron acceptors.J Bacteriol. 2005 Mar;187(5):1659-67. doi: 10.1128/JB.187.5.1659-1667.2005. J Bacteriol. 2005. PMID: 15716436 Free PMC article.
-
Anaerobic Metabolism in Haloferax Genus: Denitrification as Case of Study.Adv Microb Physiol. 2016;68:41-85. doi: 10.1016/bs.ampbs.2016.02.001. Epub 2016 Mar 15. Adv Microb Physiol. 2016. PMID: 27134021 Review.
-
Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors.Biochim Biophys Acta. 1997 Jul 4;1320(3):217-34. doi: 10.1016/s0005-2728(97)00034-0. Biochim Biophys Acta. 1997. PMID: 9230919 Review.
Cited by
-
Genome-wide transcriptional response to silver stress in extremely halophilic archaeon Haloferax alexandrinus DSM 27206 T.BMC Microbiol. 2023 Dec 4;23(1):381. doi: 10.1186/s12866-023-03133-z. BMC Microbiol. 2023. PMID: 38049746 Free PMC article.
-
Pharmaceutical applications of halophilic enzymes.Heliyon. 2025 Feb 17;11(4):e42754. doi: 10.1016/j.heliyon.2025.e42754. eCollection 2025 Feb 28. Heliyon. 2025. PMID: 40066035 Free PMC article. Review.
-
Analysis of the external signals driving the transcriptional regulation of the main genes involved in denitrification in Haloferax mediterranei.Front Microbiol. 2023 Mar 16;14:1109550. doi: 10.3389/fmicb.2023.1109550. eCollection 2023. Front Microbiol. 2023. PMID: 37007523 Free PMC article.
-
Comparative genomics of the highly halophilic Haloferacaceae.Sci Rep. 2024 Nov 6;14(1):27025. doi: 10.1038/s41598-024-78438-8. Sci Rep. 2024. PMID: 39506039 Free PMC article.
-
Gene expression analysis of Alcaligenes faecalis during induction of heterotrophic nitrification.Sci Rep. 2021 Nov 29;11(1):23105. doi: 10.1038/s41598-021-02579-3. Sci Rep. 2021. PMID: 34845321 Free PMC article.
References
-
- Hartman AH, Norais C, Badger JH, Delmas S, Haldenby S, Madupu R, Robinson J, Khouri H, Ren Q, Lowe TM, Maupin-Furlow J, Pohlschroder M, Daniels C, Pfeiffer F, Allers T, Eisen JA. 2010. The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS One 5:e9605. doi:10.1371/journal.pone.0009605. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

