Neonatal Enteropathogenic Escherichia coli Infection Disrupts Microbiota-Gut-Brain Axis Signaling
- PMID: 33820817
- PMCID: PMC8370682
- DOI: 10.1128/IAI.00059-21
Neonatal Enteropathogenic Escherichia coli Infection Disrupts Microbiota-Gut-Brain Axis Signaling
Abstract
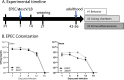
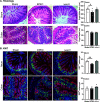
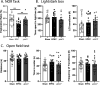
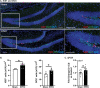
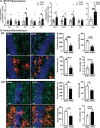
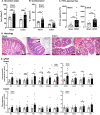
Diarrheal diseases are a leading cause of death in children under the age of 5 years worldwide. Repeated early-life exposures to diarrheal pathogens can result in comorbidities including stunted growth and cognitive deficits, suggesting an impairment in the microbiota-gut-brain (MGB) axis. Neonatal C57BL/6 mice were infected with enteropathogenic Escherichia coli (EPEC) (strain e2348/69; ΔescV [type III secretion system {T3SS} mutant]) or the vehicle (Luria-Bertani [LB] broth) via orogastric gavage at postnatal day 7 (P7). Behavior (novel-object recognition [NOR] task, light/dark [L/D] box, and open-field test [OFT]), intestinal physiology (Ussing chambers), and the gut microbiota (16S Illumina sequencing) were assessed in adulthood (6 to 8 weeks of age). Neonatal infection of mice with EPEC, but not the T3SS mutant, caused ileal inflammation in neonates and impaired recognition memory (NOR task) in adulthood. Cognitive impairments were coupled with increased neurogenesis (Ki67 and doublecortin immunostaining) and neuroinflammation (increased microglia activation [Iba1]) in adulthood. Intestinal pathophysiology in adult mice was characterized by increased secretory state (short-circuit current [Isc]) and permeability (conductance) (fluorescein isothiocyanate [FITC]-dextran flux) in the ileum and colon of neonatally EPEC-infected mice, along with increased expression of proinflammatory cytokines (Tnfα, Il12, and Il6) and pattern recognition receptors (Nod1/2 and Tlr2/4). Finally, neonatal EPEC infection caused significant dysbiosis of the gut microbiota, including decreased Firmicutes, in adulthood. Together, these findings demonstrate that infection in early life can significantly impair the MGB axis in adulthood.
Keywords: bacterial infection; behavior; microbiota-gut-brain axis; neurogenesis; neuroinflammation.
Figures


References
-
- Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. 2013. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 8:e72788. 10.1371/journal.pone.0072788. - DOI - PMC - PubMed
-
- MAL-ED Network Investigators. 2014. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 59(Suppl 4):S193–S206. 10.1093/cid/ciu653. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

