The RD2 Pathogenicity Island Modifies the Disease Potential of the Group A Streptococcus
- PMID: 33820819
- PMCID: PMC8281230
- DOI: 10.1128/IAI.00722-20
The RD2 Pathogenicity Island Modifies the Disease Potential of the Group A Streptococcus
Abstract
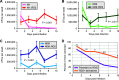
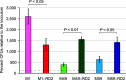
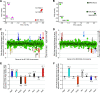
Serotype M28 isolates of the group A Streptococcus (GAS; Streptococcus pyogenes) are nonrandomly associated with cases of puerperal sepsis, a potentially life-threatening infection that can occur in women following childbirth. Previously, we discovered that the 36.3-kb RD2 pathogenicity island, which is present in serotype M28 isolates but lacking from most other isolates, promotes the ability of M28 GAS to colonize the female reproductive tract. Here, we performed a gain-of-function study in which we introduced RD2 into representative serotype M1, M49, and M59 isolates and assessed the phenotypic consequences of RD2 acquisition. All RD2-containing derivatives colonized a higher percentage of mice, and at higher CFU levels, than did the parental isolates in a mouse vaginal colonization model. However, for two additional phenotypes, survival in heparinized whole human blood and adherence to two human vaginal epithelial cell lines, there were serotype-specific differences from RD2 acquisition. Using transcriptomic comparisons, we identified that such differences may be a consequence of RD2 altering the abundance of transcripts from select core genome genes along serotype-specific lines. Our study is the first that interrogates RD2 function in GAS serotypes other than M28 isolates, shedding light on variability in the phenotypic consequences of RD2 acquisition and informing on why this mobile genetic element is not ubiquitous in the GAS population.
Keywords: Streptococcus pyogenes; mobile genetic elements; virulence.
Figures



Similar articles
-
The Mobile Genetic Element RD2 Affects Colonization Potential of Different GAS Serotypes.Infect Immun. 2021 Jul 15;89(8):e0018521. doi: 10.1128/IAI.00185-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 33972369 Free PMC article.
-
A Mobile Genetic Element Promotes the Association Between Serotype M28 Group A Streptococcus Isolates and Cases of Puerperal Sepsis.J Infect Dis. 2019 Jul 31;220(5):882-891. doi: 10.1093/infdis/jiz195. J Infect Dis. 2019. PMID: 31107945 Free PMC article.
-
The group A Streptococcus pathogenicity island RD2: virulence role and barriers to conjugative transfer.Infect Immun. 2025 Jan 31;93(1):e0027324. doi: 10.1128/iai.00273-24. Epub 2024 Nov 27. Infect Immun. 2025. PMID: 39601571 Free PMC article.
-
Human disease isolates of serotype m4 and m22 group a streptococcus lack genes required for hyaluronic acid capsule biosynthesis.mBio. 2012 Nov 6;3(6):e00413-12. doi: 10.1128/mBio.00413-12. mBio. 2012. PMID: 23131832 Free PMC article. Review.
-
Regulatory gene mutation: a driving force behind group a Streptococcus strain- and serotype-specific variation.Mol Microbiol. 2017 Feb;103(4):576-589. doi: 10.1111/mmi.13584. Epub 2016 Dec 19. Mol Microbiol. 2017. PMID: 27868255 Free PMC article. Review.
Cited by
-
Identification of distinct impacts of CovS inactivation on the transcriptome of acapsular group A streptococci.mSystems. 2023 Aug 31;8(4):e0022723. doi: 10.1128/msystems.00227-23. Epub 2023 Jun 26. mSystems. 2023. PMID: 37358280 Free PMC article.
-
The Integrative Conjugative Element ICESpyM92 Contributes to Pathogenicity of Emergent Antimicrobial-Resistant emm92 Group A Streptococcus.Infect Immun. 2022 Aug 18;90(8):e0008022. doi: 10.1128/iai.00080-22. Epub 2022 Aug 1. Infect Immun. 2022. PMID: 35913172 Free PMC article.
-
ICESp1109, a Novel Hybrid Integrative Conjugative Element of Macrolide-Resistant Streptococcus pyogenes Serotype M77 Collected Between 2003 and 2017 in Poland.J Infect Dis. 2025 Mar 17;231(3):e521-e530. doi: 10.1093/infdis/jiae473. J Infect Dis. 2025. PMID: 39393813 Free PMC article.
-
The Mobile Genetic Element RD2 Affects Colonization Potential of Different GAS Serotypes.Infect Immun. 2021 Jul 15;89(8):e0018521. doi: 10.1128/IAI.00185-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 33972369 Free PMC article.
-
Characterization of M-Type-Specific Pilus Expression in Group A Streptococcus.J Bacteriol. 2022 Nov 15;204(11):e0027022. doi: 10.1128/jb.00270-22. Epub 2022 Oct 26. J Bacteriol. 2022. PMID: 36286511 Free PMC article.
References
-
- Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, Pichon B, Baker S, Parry CM, Lambertsen LM, Shahinas D, Pillai DR, Mitchell TJ, Dougan G, Tomasz A, Klugman KP, Parkhill J, Hanage WP, Bentley SD. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331:430–434. 10.1126/science.1198545. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

