Impacts of biodiversity and biodiversity loss on zoonotic diseases
- PMID: 33820825
- PMCID: PMC8092607
- DOI: 10.1073/pnas.2023540118
Impacts of biodiversity and biodiversity loss on zoonotic diseases
Abstract
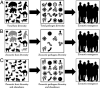
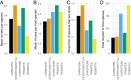
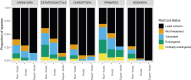
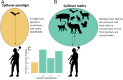
Zoonotic diseases are infectious diseases of humans caused by pathogens that are shared between humans and other vertebrate animals. Previously, pristine natural areas with high biodiversity were seen as likely sources of new zoonotic pathogens, suggesting that biodiversity could have negative impacts on human health. At the same time, biodiversity has been recognized as potentially benefiting human health by reducing the transmission of some pathogens that have already established themselves in human populations. These apparently opposing effects of biodiversity in human health may now be reconcilable. Recent research demonstrates that some taxa are much more likely to be zoonotic hosts than others are, and that these animals often proliferate in human-dominated landscapes, increasing the likelihood of spillover. In less-disturbed areas, however, these zoonotic reservoir hosts are less abundant and nonreservoirs predominate. Thus, biodiversity loss appears to increase the risk of human exposure to both new and established zoonotic pathogens. This new synthesis of the effects of biodiversity on zoonotic diseases presents an opportunity to articulate the next generation of research questions that can inform management and policy. Future studies should focus on collecting and analyzing data on the diversity, abundance, and capacity to transmit of the taxa that actually share zoonotic pathogens with us. To predict and prevent future epidemics, researchers should also focus on how these metrics change in response to human impacts on the environment, and how human behaviors can mitigate these effects. Restoration of biodiversity is an important frontier in the management of zoonotic disease risk.
Keywords: biodiversity; disease; disease ecology; zoonoses; zoonotic disease.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Centers for Disease Control and Prevention , MicrobeNet (2020). https://www.cdc.gov/microbenet/index.html. Accessed 24 August 2020.
-
- Janbon G., Quintin J., Lanternier F., d’Enfert C., Studying fungal pathogens of humans and fungal infections: Fungal diversity and diversity of approaches. Genes Immun. 20, 403–414 (2019). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

