Systems Approach to Discovery of Therapeutic Targets for Vein Graft Disease: PPARα Pivotally Regulates Metabolism, Activation, and Heterogeneity of Macrophages and Lesion Development
- PMID: 33821665
- PMCID: PMC8212880
- DOI: 10.1161/CIRCULATIONAHA.119.043724
Systems Approach to Discovery of Therapeutic Targets for Vein Graft Disease: PPARα Pivotally Regulates Metabolism, Activation, and Heterogeneity of Macrophages and Lesion Development
Abstract
Background: Vein graft failure remains a common clinical challenge. We applied a systems approach in mouse experiments to discover therapeutic targets for vein graft failure.
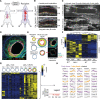
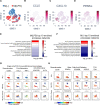
Methods: Global proteomics and high-dimensional clustering on multiple vein graft tissues were used to identify potential pathogenic mechanisms. The PPARs (peroxisome proliferator-activated receptors) pathway served as an example to substantiate our discovery platform. In vivo mouse experiments with macrophage-targeted PPARα small interfering RNA, or the novel, selective activator pemafibrate demonstrate the role of PPARα in the development and inflammation of vein graft lesions. In vitro experiments further included metabolomic profiling, quantitative polymerase chain reaction, flow cytometry, metabolic assays, and single-cell RNA sequencing on primary human and mouse macrophages.
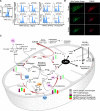
Results: We identified changes in the vein graft proteome associated with immune responses, lipid metabolism regulated by the PPARs, fatty acid metabolism, matrix remodeling, and hematopoietic cell mobilization. PPARα agonism by pemafibrate retarded the development and inflammation of vein graft lesions in mice, whereas gene silencing worsened plaque formation. Pemafibrate also suppressed arteriovenous fistula lesion development. Metabolomics/lipidomics, functional metabolic assays, and single-cell analysis of cultured human macrophages revealed that PPARα modulates macrophage glycolysis, citrate metabolism, mitochondrial membrane sphingolipid metabolism, and heterogeneity.
Conclusions: This study explored potential drivers of vein graft inflammation and identified PPARα as a novel potential pharmacological treatment for this unmet medical need.
Keywords: arteriovenous; fistula; inflammation; macrophage; pemafibrate; proteomics; systems biology; vein graft.
Figures




Comment in
-
PPARα regulates the development and inflammation of vein graft lesions.Nat Rev Cardiol. 2021 Jun;18(6):386. doi: 10.1038/s41569-021-00556-x. Nat Rev Cardiol. 2021. PMID: 33879845 No abstract available.
-
Integrative Multiomics Approaches for Discovery of New Drug Targets for Cardiovascular Disease.Circulation. 2021 Jun 22;143(25):2471-2474. doi: 10.1161/CIRCULATIONAHA.121.054900. Epub 2021 Jun 21. Circulation. 2021. PMID: 34152794 Free PMC article. No abstract available.
References
-
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 - PubMed
-
- Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–350. doi: 10.1161/CIRCULATIONAHA.117.032235 - PubMed
-
- Klingelhoefer E, Bergert H, Kersting S, Ludwig S, Weiss N, Schönleben F, Grützmann R, Gäbel G. Predictive factors for better bypass patency and limb salvage after prosthetic above-knee bypass reconstruction. J Vasc Surg. 2016;64:380–388.e1. doi: 10.1016/j.jvs.2016.02.059 - PubMed
-
- Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W; ACT I Investigators. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374:1011–1020. doi: 10.1056/NEJMoa1515706 - PubMed
-
- Conte MS. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) and the (hoped for) dawn of evidence-based treatment for advanced limb ischemia. J Vasc Surg. 2010;51(5 suppl):69S–75S. doi: 10.1016/j.jvs.2010.02.001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

