Discovery and characterization of a novel family of prokaryotic nanocompartments involved in sulfur metabolism
- PMID: 33821786
- PMCID: PMC8049743
- DOI: 10.7554/eLife.59288
Discovery and characterization of a novel family of prokaryotic nanocompartments involved in sulfur metabolism
Abstract
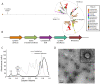
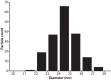
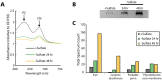
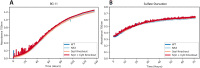
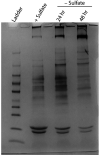
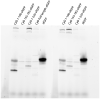
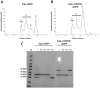
Prokaryotic nanocompartments, also known as encapsulins, are a recently discovered proteinaceous organelle-like compartment in prokaryotes that compartmentalize cargo enzymes. While initial studies have begun to elucidate the structure and physiological roles of encapsulins, bioinformatic evidence suggests that a great diversity of encapsulin nanocompartments remains unexplored. Here, we describe a novel encapsulin in the freshwater cyanobacterium Synechococcus elongatus PCC 7942. This nanocompartment is upregulated upon sulfate starvation and encapsulates a cysteine desulfurase enzyme via an N-terminal targeting sequence. Using cryo-electron microscopy, we have determined the structure of the nanocompartment complex to 2.2 Å resolution. Lastly, biochemical characterization of the complex demonstrated that the activity of the cysteine desulfurase is enhanced upon encapsulation. Taken together, our discovery, structural analysis, and enzymatic characterization of this prokaryotic nanocompartment provide a foundation for future studies seeking to understand the physiological role of this encapsulin in various bacteria.
Keywords: E. coli; Synechococcus elongatus; cryo-EM; encapsulin; infectious disease; microbiology; molecular biophysics; nanocompartment; prokaryotic organelle; structural biology; sulfur starvation.
© 2021, Nichols et al.
Conflict of interest statement
RN, BL, NP, DR, LO, LV, AB, EN, DS No competing interests declared
Figures








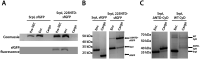
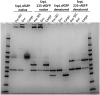

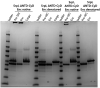




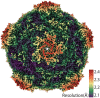





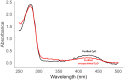
References
-
- Abdellrazeq GS, Elnaggar MM, Bannantine JP, Park KT, Souza CD, Backer B, Hulubei V, Fry LM, Khaliel SA, Torky HA, Schneider DA, Davis WC. A Mycobacterium avium subsp paratuberculosis relA deletion mutant and a 35 kDa major membrane protein elicit development of cytotoxic T lymphocytes with ability to kill intracellular bacteria. Veterinary Research. 2018;49:53. doi: 10.1186/s13567-018-0549-3. - DOI - PMC - PubMed
-
- Abdellrazeq GS, Fry LM, Elnaggar MM, Bannantine JP, Schneider DA, Chamberlin WM, Mahmoud AHA, Park KT, Hulubei V, Davis WC. Simultaneous cognate epitope recognition by bovine CD4 and CD8 T cells is essential for primary expansion of antigen-specific cytotoxic T-cells following ex vivo stimulation with a candidate Mycobacterium avium subsp paratuberculosis peptide vaccine. Vaccine. 2020;38:2016–2025. doi: 10.1016/j.vaccine.2019.12.052. - DOI - PubMed
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Akita F, Chong KT, Tanaka H, Yamashita E, Miyazaki N, Nakaishi Y, Suzuki M, Namba K, Ono Y, Tsukihara T, Nakagawa A. The crystal structure of a Virus-like particle from the hyperthermophilic archaeon Pyrococcus furiosus provides insight into the evolution of viruses. Journal of Molecular Biology. 2007;368:1469–1483. doi: 10.1016/j.jmb.2007.02.075. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

