Potential Role of Epithelial Endoplasmic Reticulum Stress and Anterior Gradient Protein 2 Homologue in Crohn's Disease Fibrosis
- PMID: 33822017
- PMCID: PMC8861373
- DOI: 10.1093/ecco-jcc/jjab061
Potential Role of Epithelial Endoplasmic Reticulum Stress and Anterior Gradient Protein 2 Homologue in Crohn's Disease Fibrosis
Abstract
Background and aims: Intestinal fibrosis is a common complication of Crohn's disease [CD]. It is characterised by an accumulation of fibroblasts differentiating into myofibroblasts secreting excessive extracellular matrix. The potential role of the intestinal epithelium in this fibrotic process remains poorly defined.
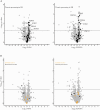
Methods: We performed a pilot proteomic study comparing the proteome of surface epithelium, isolated by laser-capture microdissection, in normal and fibrotic zones of resected ileal CD strictures [13 zones collected in five patients]. Proteins of interests were validated by immunohistochemistry [IHC] in ileal and colonic samples of stricturing CD [n = 44], pure inflammatory CD [n = 29], and control [n = 40] subjects. The pro-fibrotic role of one selected epithelial protein was investigated through in-vitro experiments using HT-29 epithelial cells and a CCD-18Co fibroblast to myofibroblast differentiation model.
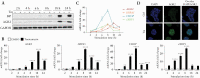
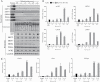
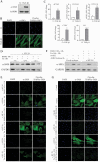
Results: Proteomic study revealed an endoplasmic reticulum [ER] stress proteins increase in the epithelium of CD ileal fibrotic strictures, including anterior gradient protein 2 homologue [AGR2] and binding-immunoglobulin protein [BiP]. This was confirmed by IHC. In HT-29 cells, tunicamycin-induced ER stress triggered AGR2 intracellular expression and its secretion. Supernatant of these HT-29 cells, pre-conditioned by tunicamycin, led to a myofibroblastic differentiation when applied on CCD-18Co fibroblasts. By using recombinant protein and blocking agent for AGR2, we demonstrated that the secretion of this protein by epithelial cells can play a role in the myofibroblastic differentiation.
Conclusions: The development of CD fibrotic strictures could involve epithelial ER stress and particularly the secretion of AGR2.
Keywords: CD fibrosis; ER stress; anterior gradient protein 2 homologue.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis 2017;23:133–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

