Comparative Efficacy of Cabozantinib and Ramucirumab After Sorafenib for Patients with Hepatocellular Carcinoma and Alpha-fetoprotein ≥ 400 ng/mL: A Matching-Adjusted Indirect Comparison
- PMID: 33822328
- PMCID: PMC8107171
- DOI: 10.1007/s12325-021-01700-2
Comparative Efficacy of Cabozantinib and Ramucirumab After Sorafenib for Patients with Hepatocellular Carcinoma and Alpha-fetoprotein ≥ 400 ng/mL: A Matching-Adjusted Indirect Comparison
Abstract
Introduction: Cabozantinib and ramucirumab are approved for the treatment of adults with hepatocellular carcinoma (HCC) following prior sorafenib treatment; ramucirumab is restricted to use in patients with serum alpha-fetoprotein (AFP) ≥ 400 ng/mL. This matching-adjusted indirect comparison evaluated the efficacy and safety of both drugs after sorafenib in patients with HCC and AFP ≥ 400 ng/mL.
Methods: Individual patient data (IPD) from the CELESTIAL trial (cabozantinib) and population-level data from the REACH-2 trial (ramucirumab) were used. To align with REACH-2, the CELESTIAL population was limited to patients who received first-line sorafenib only and had baseline serum AFP ≥ 400 ng/mL. The IPD from CELESTIAL were weighted to balance the distribution of 11 effect-modifying baseline characteristics with those of REACH-2. Overall survival (OS; primary endpoint) and progression-free survival (PFS) were compared for the CELESTIAL (matching-adjusted) and REACH-2 populations using weighted Kaplan-Meier (KM) curves and parametric (OS, Weibull; PFS, log-logistic) modeling. Rates of treatment-related adverse events (TRAEs) and TRAE-related discontinuations were also compared.
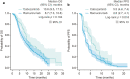
Results: After matching and weighting, baseline characteristics were balanced between populations (REACH-2, N = 292; CELESTIAL, effective sample size = 105). Weighted KM estimates for OS (median [95% CI]) were not significantly different between cabozantinib and ramucirumab (10.6 [9.5-17.3] months versus 8.7 [7.3-10.8] months; p = 0.104), but PFS was significantly longer for cabozantinib than for ramucirumab (5.5 [4.6-7.4] months versus 2.8 [2.7-4.1] months; p = 0.016). Parametric modeling results were consistent with the weighted KM analysis. Rates of some grade 3 or 4 TRAEs were lower with ramucirumab than with cabozantinib; however, TRAE-related discontinuation rates were similar (p = 0.271).
Conclusion: In this MAIC, cabozantinib significantly prolonged median PFS compared with ramucirumab after prior sorafenib treatment in patients with HCC and AFP ≥ 400 ng/mL; rates of some grade 3 or 4 TRAEs were lower with ramucirumab than cabozantinib but related discontinuation rates were not significantly different between treatments.
Trial registration: Clinical trials.gov identifiers: CELESTIAL trial (NCT01908426) and REACH-2 trial (NCT02435433). These slides can be retrieved under Electronic Supplementary Material.
Keywords: Alpha-fetoprotein (AFP); Cabozantinib; Hepatocellular carcinoma (HCC); Indirect treatment comparison (ITC); Matching-adjusted indirect comparison (MAIC); Monoclonal antibody (mAb); Ramucirumab; Tyrosine kinase inhibitor (TKI); Vascular endothelial growth factor (VEGF); second-line treatment / 2L treatment.
Figures
References
-
- Exelixis I. Prescribing information: CABOMETYX® (cabozantinib) tablets, for oral use. US Food and Drug Administration, 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208692s003lbl.pdf. Accessed 2 Dec 2020.
-
- Ipsen Pharma. Summary of product characteristics, CABOMETYX. European Medicines Agency, 2020. https://www.ema.europa.eu/en/documents/product-information/cabometyx-epa.... Accessed 2 Dec 2020.
-
- Bayer AG. Summary of product characteristics, Stivarga 40 mg film-coated tablets. European Medicines Agency, 2018. Available from: https://www.ema.europa.eu/en/documents/product-information/stivarga-epar.... Accessed 2 Dec 2020.
-
- Bayer HealthCare Pharmaceuticals Inc. Prescribing Information: STIVARGA® (regorafenib) tablets, for oral use. US Food and Drug Administration, 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203085s007lbl.pdf. Accessed 2 Dec 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical