Treatment of Pediatric Adrenocortical Carcinoma With Surgery, Retroperitoneal Lymph Node Dissection, and Chemotherapy: The Children's Oncology Group ARAR0332 Protocol
- PMID: 33822640
- PMCID: PMC8462560
- DOI: 10.1200/JCO.20.02871
Treatment of Pediatric Adrenocortical Carcinoma With Surgery, Retroperitoneal Lymph Node Dissection, and Chemotherapy: The Children's Oncology Group ARAR0332 Protocol
Abstract
Purpose: Adrenocortical carcinoma (ACC) is a rare aggressive pediatric malignancy with distinct biology. Its treatment follows the principles developed for adults; pediatric-specific studies are scarce.
Patients and methods: Prospective single-arm risk-stratified interventional study. Study objectives were (1) to describe the outcome of patients with stage I ACC treated with adrenalectomy alone; (2) to describe the outcome of stage II patients (completely resected > 200 cc or > 100 g) treated with adrenalectomy and retroperitoneal lymph node dissection; and (3) to describe the outcome of patients with stage III or IV treated with mitotane and chemotherapy.
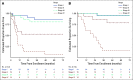
Results: Between September 2006 and May 2013, 78 patients (77 eligible, 51 females) were enrolled. The 5-year event-free survival estimates for stages I (24 patients), II (15 patients), III (24 patients), and IV (14 patients) were 86.2%, 53.3%, 81%, and 7.1%, respectively. The corresponding 5-year overall survival estimates were 95.2%, 78.8%, 94.7%, and 15.6%, respectively. On univariate analysis, age, stage, presence of virilization, Cushing syndrome, or hypertension, germline TP53 status, and presence of a somatic ATRX mutation were associated with outcome. On multivariable analysis, only stage and age were significantly associated with outcome. The probabilities of mitotane and chemotherapy feasibility events were 10.5% and 31.6%, respectively.
Conclusion: Outcome for children with stage I ACC is excellent with surgery. Outcome for patients with stage II disease is inferior despite retroperitoneal lymph node dissection. Patients with stage III ACC have an excellent outcome combining surgery and chemotherapy. Patients with stage IV ACC are older and have a poor outcome; new treatments should be explored for this high-risk group. The combination of mitotane and chemotherapy as prescribed in ARAR0332 resulted in significant toxicity; one third of patients with advanced disease could not complete the scheduled treatment.
Conflict of interest statement
Figures
Comment in
-
Reply to J.-G. Wang et al.J Clin Oncol. 2021 Sep 20;39(27):3088-3089. doi: 10.1200/JCO.21.01389. Epub 2021 Jul 6. J Clin Oncol. 2021. PMID: 34228509 No abstract available.
-
Regarding the Children's Oncology Group ARAR0332 Protocol for Pediatric Adrenocortical Carcinoma.J Clin Oncol. 2021 Sep 20;39(27):3087-3088. doi: 10.1200/JCO.21.00918. Epub 2021 Jul 6. J Clin Oncol. 2021. PMID: 34228512 No abstract available.
-
Different management of adrenocortical carcinoma in children compared to adults: is it time to share guidelines?Endocrine. 2021 Dec;74(3):475-477. doi: 10.1007/s12020-021-02874-z. Epub 2021 Sep 24. Endocrine. 2021. PMID: 34559356 Free PMC article.
References
-
- Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors. An analysis of 254 cases from the International Pediatric Adrenocortical Tumor Registry J Clin Oncol 22838–8452004 - PubMed
-
- Ribeiro RC, Pinto EM, Zambetti GP, et al. The International Pediatric Adrenocortical Tumor Registry initiative: Contributions to clinical, biological, and treatment advances in pediatric adrenocortical tumors Mol Cell Endocrinol 35137–432012 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous