SARS-CoV-2 vaccines: a triumph of science and collaboration
- PMID: 33822773
- PMCID: PMC8262277
- DOI: 10.1172/jci.insight.149187
SARS-CoV-2 vaccines: a triumph of science and collaboration
Abstract
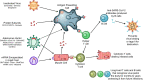
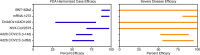
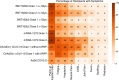
Roughly 1 year after the first case of COVID-19 was identified and less than 1 year after the sequencing of SARS-CoV-2, multiple SARS-CoV-2 vaccines with demonstrated safety and efficacy in phase III clinical trials are available. The most promising vaccines have targeted the surface glycoprotein (S-protein) of SARS-CoV-2 and achieved an approximate 85%-95% reduction in the risk of symptomatic COVID-19, while retaining excellent safety profiles and modest side effects in the phase III clinical trials. The mRNA, replication-incompetent viral vector, and protein subunit vaccine technologies have all been successfully employed. Some novel SARS-CoV-2 variants evade but do not appear to fully overcome the potent immunity induced by these vaccines. Emerging real-world effectiveness data add evidence for protection from severe COVID-19. This is an impressive first demonstration of the effectiveness of the mRNA vaccine and vector vaccine platforms. The success of SARS-CoV-2 vaccine development should be credited to open science, industry partnerships, harmonization of clinical trials, and the altruism of study participants. The manufacturing and distribution of the emergency use-authorized SARS-CoV-2 vaccines are ongoing challenges. What remains now is to ensure broad and equitable global vaccination against COVID-19.
Conflict of interest statement
Figures
References
-
- World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... Updated April 2, 2021. Accessed March 18, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

