Natural mucosal barriers and COVID-19 in children
- PMID: 33822777
- PMCID: PMC8262299
- DOI: 10.1172/jci.insight.148694
Natural mucosal barriers and COVID-19 in children
Abstract
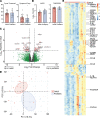
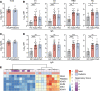
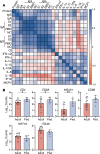
BACKGROUNDCoronavirus disease 2019 (COVID-19) is more benign in children compared with adults for unknown reasons. This contrasts with other respiratory viruses where disease manifestations are often more severe in children. We hypothesize that a more robust early innate immune response to SARS coronavirus 2 (SARS-CoV-2) protects against severe disease.METHODSClinical outcomes, SARS-CoV-2 viral copies, and cellular gene expression were compared in nasopharyngeal swabs obtained at the time of presentation to the emergency department from 12 children and 27 adults using bulk RNA sequencing and quantitative reverse-transcription PCR. Total protein, cytokines, and anti-SARS-CoV-2 IgG and IgA were quantified in nasal fluid.RESULTSSARS-CoV-2 copies, angiotensin-converting enzyme 2, and TMPRSS2 gene expression were similar in children and adults, but children displayed higher expression of genes associated with IFN signaling, NLRP3 inflammasome, and other innate pathways. Higher levels of IFN-α2, IFN-γ, IP-10, IL-8, and IL-1β protein were detected in nasal fluid in children versus adults. Children also expressed higher levels of genes associated with immune cells, whereas expression of those associated with epithelial cells did not differ in children versus adults. Anti-SARS-CoV-2 IgA and IgG were detected at similar levels in nasal fluid from both groups. None of the children required supplemental oxygen, whereas 7 adults did (P = 0.03); 4 adults died.CONCLUSIONThese findings provide direct evidence of a more vigorous early mucosal immune response in children compared with adults and suggest that this contributes to favorable clinical outcomes.FUNDINGNIH grants R01 AI134367, UL1 TR002556, T32 AI007501, T32GM007288, P30 AI124414; an Albert Einstein College of Medicine Dean's COVID-19 Pilot Research Award; and the Eric J. Heyer, MD, PhD Translational Research Pilot Project Award.
Keywords: Infectious disease; Innate immunity.
Conflict of interest statement
Figures

Update of
-
Natural Mucosal Barriers and COVID-19 in Children.medRxiv [Preprint]. 2021 Feb 13:2021.02.12.21251310. doi: 10.1101/2021.02.12.21251310. medRxiv. 2021. Update in: JCI Insight. 2021 May 10;6(9):148694. doi: 10.1172/jci.insight.148694. PMID: 33594377 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

