Tim-3 is dispensable for allergic inflammation and respiratory tolerance in experimental asthma
- PMID: 33822811
- PMCID: PMC8023500
- DOI: 10.1371/journal.pone.0249605
Tim-3 is dispensable for allergic inflammation and respiratory tolerance in experimental asthma
Abstract
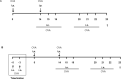
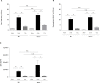
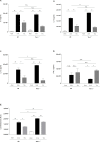
T cell immunoglobulin and mucin domain-containing molecule-3 (Tim-3) has been described as a transmembrane protein, expressed on the surface of various T cells as well as different cells of innate immunity. It has since been associated with Th1 mediated autoimmune diseases and transplantation tolerance studies, thereby indicating a possible role of this receptor in counter-regulation of Th2 immune responses. In the present study we therefore directly examined the role of Tim-3 in allergic inflammation and respiratory tolerance. First, Tim-3-/- mice and wild type controls were immunized and challenged with the model allergen ovalbumin (OVA) to induce an asthma-like phenotype. Analysis of cell numbers and distribution in the bronchoalveolar lavage (BAL) fluid as well as lung histology in H&E stained lung sections demonstrated a comparable degree of eosinophilic inflammation in both mouse strains. Th2 cytokine production in restimulated cell culture supernatants and serum IgE and IgG levels were equally increased in both genotypes. In addition, cell proliferation and the distribution of different T cell subsets were comparable. Moreover, analysis of both mouse strains in our respiratory tolerance model, where mucosal application of the model allergen before immunization, prevents the development of an asthma-like phenotype, revealed no differences in any of the parameters mentioned above. The current study demonstrates that Tim-3 is dispensable not only for the development of allergic inflammation but also for induction of respiratory tolerance in mice in an OVA-based model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
CD137 deficiency does not affect development of airway inflammation or respiratory tolerance induction in murine models.Clin Exp Immunol. 2012 Jun;168(3):308-17. doi: 10.1111/j.1365-2249.2012.04572.x. Clin Exp Immunol. 2012. PMID: 22519594 Free PMC article.
-
TIM-3 is not essential for development of airway inflammation induced by house dust mite antigens.Allergol Int. 2016 Oct;65(4):459-465. doi: 10.1016/j.alit.2016.04.008. Epub 2016 May 18. Allergol Int. 2016. PMID: 27209052 Free PMC article.
-
Pneumococcal pneumonia suppresses allergy development but preserves respiratory tolerance in mice.Immunol Lett. 2015 Mar;164(1):44-52. doi: 10.1016/j.imlet.2014.12.001. Epub 2015 Jan 6. Immunol Lett. 2015. PMID: 25576460
-
Significance of persistent inflammation in respiratory disorders induced by nanoparticles.J Immunol Res. 2014;2014:962871. doi: 10.1155/2014/962871. Epub 2014 Jul 7. J Immunol Res. 2014. PMID: 25097864 Free PMC article. Review.
-
The emerging role of the TIM molecules in transplantation.Am J Transplant. 2011 Oct;11(10):2012-9. doi: 10.1111/j.1600-6143.2011.03727.x. Epub 2011 Sep 11. Am J Transplant. 2011. PMID: 21906254 Free PMC article. Review.
Cited by
-
The Putative Role of TIM-3 Variants in Polyendocrine Autoimmunity: Insights from a WES Investigation.Int J Mol Sci. 2024 Oct 12;25(20):10994. doi: 10.3390/ijms252010994. Int J Mol Sci. 2024. PMID: 39456777 Free PMC article.
-
TIM-3-driven macrophage polarisation is associated to recalcitrant chronic rhinosinusitis with nasal polyps.Acta Otorhinolaryngol Ital. 2024 Aug;44(4):242-251. doi: 10.14639/0392-100X-N2717. Acta Otorhinolaryngol Ital. 2024. PMID: 39347549 Free PMC article.
-
CAR-NKT Cells in Asthma: Use of NKT as a Promising Cell for CAR Therapy.Clin Rev Allergy Immunol. 2024 Jun;66(3):328-362. doi: 10.1007/s12016-024-08998-0. Epub 2024 Jul 12. Clin Rev Allergy Immunol. 2024. PMID: 38995478 Review.
References
-
- Robinson Douglas S., MD FRCP. The role of the T cell in asthma. Journal of Allergy and Clinical Immunology, The 2010;126(6):1081–1091. - PubMed
-
- Hartl D, MD, Koller B, MD, Mehlhorn AT, MD, Reinhardt D, MD, Nicolai T, MD, Schendel DJ, PhD, et al.. Quantitative and functional impairment of pulmonary CD4+ CD25hi regulatory T cells in pediatric asthma. Journal of Allergy and Clinical Immunology, The 2007;119(5):1258–1266. 10.1016/j.jaci.2007.02.023 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

