Heparin binding protein in severe COVID-19-A prospective observational cohort study
- PMID: 33822821
- PMCID: PMC8023466
- DOI: 10.1371/journal.pone.0249570
Heparin binding protein in severe COVID-19-A prospective observational cohort study
Abstract
Background and aims: Neutrophil-derived heparin binding protein (HBP; also known as azurocidin or CAP-37) is a key player in bacterial sepsis and a promising biomarker in severe infections. The aims of this study were to assess whether HBP is involved in the pathophysiology of COVID-19 and, if so, whether it can be used to predict severe disease preferably using a point-of-care test.
Methods: This was a prospective convenience sample study of biomarkers in patients admitted to Skåne University hospital in Sweden with a confirmed COVID-19 diagnosis. Plasma samples and clinical data were collected within 72h after admission, during hospital stay and at discharge. Plasma HBP concentrations samples were measured both with enzyme-linked immunosorbent assay (ELISA) and with a novel dry immunofluorescence analyzer (Joinstar) point-of-care test.
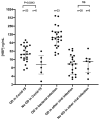
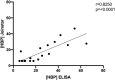
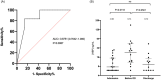
Results: Thirty-five COVID-19 patients were enrolled in the study. Twenty-nine patients had blood samples taken within 72h after admission. We compared the highest HBP value taken within 72h after admission in patients who eventually developed organ dysfunction (n = 23) compared to those who did not (n = 6), and found that HBP was significantly elevated in those who developed organ dysfunction (25.0 ng/mL (interquartile range (IQR) 16.6-48.5) vs 10.6 ng/mL (IQR 4.8-21.7 ng/mL), p = 0.03). Point-of-care test measurements correlated well with ELISA measurements (R = 0.83). HBP measured by the POC device predicted development of COVID-induced organ dysfunction with an AUC of 0.88 (95% confidence interval (CI) 0.70-1.0).
Conclusions: HBP is elevated prior to onset of organ dysfunction in patients with severe COVID-19 using a newly developed point-of-care test and hence HBP could be used in a clinical setting as a prognostic marker in COVID-19.
Conflict of interest statement
Adam Linder is listed as inventors on a patent on the use of HBP as a diagnostic tool in sepsis filed by Hansa Medical AB WO2008151808A1. This does not alter our adherence to PLOS ONE policies on sharing data and materials. All other authors have declared no relevant conflicts of interest.
Figures
References
-
- (WHO) WHO (2020) Coronavirus disease 2019 (COVID-19): situation report. World Health Organization 209.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

