An APP ectodomain mutation outside of the Aβ domain promotes Aβ production in vitro and deposition in vivo
- PMID: 33822840
- PMCID: PMC8034382
- DOI: 10.1084/jem.20210313
An APP ectodomain mutation outside of the Aβ domain promotes Aβ production in vitro and deposition in vivo
Abstract
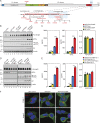
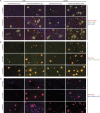
Familial Alzheimer's disease (FAD)-linked mutations in the APP gene occur either within the Aβ-coding region or immediately proximal and are located in exons 16 and 17, which encode Aβ peptides. We have identified an extremely rare, partially penetrant, single nucleotide variant (SNV), rs145081708, in APP that corresponds to a Ser198Pro substitution in exon 5. We now report that in stably transfected cells, expression of APP harboring the S198P mutation (APPS198P) leads to elevated production of Aβ peptides by an unconventional mechanism in which the folding and exit of APPS198P from the endoplasmic reticulum is accelerated. More importantly, coexpression of APP S198P and the FAD-linked PS1ΔE9 variant in the brains of male and female transgenic mice leads to elevated steady-state Aβ peptide levels and acceleration of Aβ deposition compared with age- and gender-matched mice expressing APP and PS1ΔE9. This is the first AD-linked mutation in APP present outside of exons 16 and 17 that enhances Aβ production and deposition.
© 2021 Zhang et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






Comment in
-
Alzheimer mutant speeds APP transport.J Exp Med. 2021 Jun 7;218(6):e20210511. doi: 10.1084/jem.20210511. Epub 2021 May 14. J Exp Med. 2021. PMID: 33988687 Free PMC article.
References
-
- Beecham, G.W., Bis J.C., Martin E.R., Choi S.H., DeStefano A.L., van Duijn C.M., Fornage M., Gabriel S.B., Koboldt D.C., Larson D.E., et al. . 2017. The Alzheimer’s Disease Sequencing Project: Study design and sample selection. Neurol. Genet. 3:e194. 10.1212/NXG.0000000000000194 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

