Neutrophil-Derived Tumor Necrosis Factor Drives Fungal Acute Lung Injury in Chronic Granulomatous Disease
- PMID: 33822981
- PMCID: PMC8682762
- DOI: 10.1093/infdis/jiab188
Neutrophil-Derived Tumor Necrosis Factor Drives Fungal Acute Lung Injury in Chronic Granulomatous Disease
Abstract
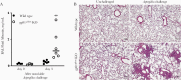
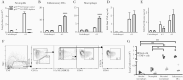
Chronic granulomatous disease (CGD) results from deficiency of nicotinamide adenine dinucleotide phosphate(NADPH) oxidase and impaired reactive oxygen species (ROS) generation. This leads to impaired killing of Aspergillus and, independently, a pathologic hyperinflammatory response to the organism. We hypothesized that neutrophil-derived ROS inhibit the inflammatory response to Aspergillus and that acute lung injury in CGD is due to failure of this regulation. Mice with gp91phox deficiency, the most common CGD mutation, had more severe lung injury, increased neutrophilinfiltration, and increased lung tumor necrosis factor (TNF) after Aspergillus challenge compared with wild-types. Neutrophils were surprisingly the predominant source of TNF in gp91phox-deficient lungs. TNF neutralization inhibited neutrophil recruitment in gp91phox-deficient mice and protected from lung injury. We propose that, in normal hosts, Aspergillus stimulates TNF-dependent neutrophil recruitment to the lungs and neutrophil-derived ROS limit inflammation. In CGD, in contrast, recruited neutrophils are the dominant source of TNF, promoting further neutrophil recruitment in a pathologic positive-feedback cycle, resulting in progressive lung injury.
Keywords: Aspergillus; TNF; chronic granulomatous disease; neutrophil; reactive oxygen species.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures




Similar articles
-
Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus.J Exp Med. 1997 Jan 20;185(2):207-18. doi: 10.1084/jem.185.2.207. J Exp Med. 1997. PMID: 9016870 Free PMC article.
-
Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease.Blood. 1997 Jan 1;89(1):41-8. Blood. 1997. PMID: 8978275
-
Does Pioglitazone Lead to Neutrophil Extracellular Traps Formation in Chronic Granulomatous Disease Patients?Front Immunol. 2019 Jul 31;10:1739. doi: 10.3389/fimmu.2019.01739. eCollection 2019. Front Immunol. 2019. PMID: 31428088 Free PMC article.
-
The roles of NADPH oxidase in modulating neutrophil effector responses.Mol Oral Microbiol. 2019 Apr;34(2):27-38. doi: 10.1111/omi.12252. Epub 2019 Feb 7. Mol Oral Microbiol. 2019. PMID: 30632295 Free PMC article. Review.
-
[Contribution of neutrophils to Aspergillus infection].Nihon Ishinkin Gakkai Zasshi. 2002;43(3):153-60. doi: 10.3314/jjmm.43.153. Nihon Ishinkin Gakkai Zasshi. 2002. PMID: 12145629 Review. Japanese.
Cited by
-
The heme scavenger hemopexin protects against lung injury during aspergillosis by mitigating release of neutrophil extracellular traps.JCI Insight. 2025 Apr 15;10(10):e189151. doi: 10.1172/jci.insight.189151. eCollection 2025 May 22. JCI Insight. 2025. PMID: 40232861 Free PMC article.
-
A Fun-Guide to Innate Immune Responses to Fungal Infections.J Fungi (Basel). 2022 Jul 29;8(8):805. doi: 10.3390/jof8080805. J Fungi (Basel). 2022. PMID: 36012793 Free PMC article. Review.
-
Aspergillus Utilizes Extracellular Heme as an Iron Source During Invasive Pneumonia, Driving Infection Severity.J Infect Dis. 2022 May 16;225(10):1811-1821. doi: 10.1093/infdis/jiac079. J Infect Dis. 2022. PMID: 35267014 Free PMC article.
-
Multi-scale mechanistic modelling of the host defence in invasive aspergillosis reveals leucocyte activation and iron acquisition as drivers of infection outcome.J R Soc Interface. 2022 Apr;19(189):20210806. doi: 10.1098/rsif.2021.0806. Epub 2022 Apr 13. J R Soc Interface. 2022. PMID: 35414216 Free PMC article.
-
Cell-Autonomous Cxcl1 Sustains Tolerogenic Circuitries and Stromal Inflammation via Neutrophil-Derived TNF in Pancreatic Cancer.Cancer Discov. 2023 Jun 2;13(6):1428-1453. doi: 10.1158/2159-8290.CD-22-1046. Cancer Discov. 2023. PMID: 36946782 Free PMC article.
References
-
- Dunogué B, Pilmis B, Mahlaoui N, et al. Chronic granulomatous disease in patients reaching adulthood: a nationwide study in France. Clin Infect Dis 2017; 64:767–75. - PubMed
-
- Winkelstein JA, Marino MC, Johnston RB Jr, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine 2000; 79:155–69. - PubMed
-
- Blumental S, Mouy R, Mahlaoui N, et al. Invasive mold infections in chronic granulomatous disease: a 25-year retrospective survey. Clin Infect Dis 2011; 53:e159–69. - PubMed
-
- Codina R, Fox RW, Lockey RF, DeMarco P, Bagg A. Typical levels of airborne fungal spores in houses without obvious moisture problems during a rainy season in Florida, USA. J Investig Allergol Clin Immunol 2008; 18:156–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

