A TOR (target of rapamycin) and nutritional phosphoproteome of fission yeast reveals novel targets in networks conserved in humans
- PMID: 33823663
- PMCID: PMC8025308
- DOI: 10.1098/rsob.200405
A TOR (target of rapamycin) and nutritional phosphoproteome of fission yeast reveals novel targets in networks conserved in humans
Abstract
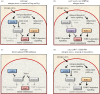
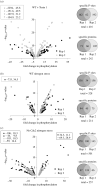
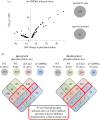
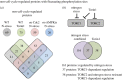
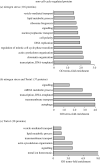
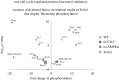
Fluctuations in TOR, AMPK and MAP-kinase signalling maintain cellular homeostasis and coordinate growth and division with environmental context. We have applied quantitative, SILAC mass spectrometry to map TOR and nutrient-controlled signalling in the fission yeast Schizosaccharomyces pombe. Phosphorylation levels at more than 1000 sites were altered following nitrogen stress or Torin1 inhibition of the TORC1 and TORC2 networks that comprise TOR signalling. One hundred and thirty of these sites were regulated by both perturbations, and the majority of these (119) new targets have not previously been linked to either nutritional or TOR control in either yeasts or humans. Elimination of AMPK inhibition of TORC1, by removal of AMPKα (ssp2::ura4+), identified phosphosites where nitrogen stress-induced changes were independent of TOR control. Using a yeast strain with an ATP analogue-sensitized Cdc2 kinase, we excluded sites that were changed as an indirect consequence of mitotic control modulation by nitrogen stress or TOR signalling. Nutritional control of gene expression was reflected in multiple targets in RNA metabolism, while significant modulation of actin cytoskeletal components points to adaptations in morphogenesis and cell integrity networks. Reduced phosphorylation of the MAPKK Byr1, at a site whose human equivalent controls docking between MEK and ERK, prevented sexual differentiation when resources were sparse but not eliminated.
Keywords: Byr1 MAPKK; TORC1; TORC2; fission yeast Schizosaccharomyces pombe; nitrogen stress; phosphoproteome.
Figures



Similar articles
-
The contribution of non-essential Schizosaccharomyces pombe genes to fitness in response to altered nutrient supply and target of rapamycin activity.Open Biol. 2018 May;8(5):180015. doi: 10.1098/rsob.180015. Open Biol. 2018. PMID: 29720420 Free PMC article.
-
Nitrogen regulates AMPK to control TORC1 signaling.Curr Biol. 2015 Feb 16;25(4):445-54. doi: 10.1016/j.cub.2014.12.034. Epub 2015 Jan 29. Curr Biol. 2015. PMID: 25639242 Free PMC article.
-
TORC1-Dependent Phosphorylation Targets in Fission Yeast.Biomolecules. 2017 Jul 3;7(3):50. doi: 10.3390/biom7030050. Biomolecules. 2017. PMID: 28671615 Free PMC article. Review.
-
Ste12/Fab1 phosphatidylinositol-3-phosphate 5-kinase is required for nitrogen-regulated mitotic commitment and cell size control.PLoS One. 2017 Mar 8;12(3):e0172740. doi: 10.1371/journal.pone.0172740. eCollection 2017. PLoS One. 2017. PMID: 28273166 Free PMC article.
-
Express yourself: how PP2A-B55Pab1 helps TORC1 talk to TORC2.Curr Genet. 2018 Feb;64(1):43-51. doi: 10.1007/s00294-017-0721-8. Epub 2017 Jun 22. Curr Genet. 2018. PMID: 28643116 Free PMC article. Review.
Cited by
-
Rapamycin-sensitive mechanisms confine the growth of fission yeast below the temperatures detrimental to cell physiology.iScience. 2023 Dec 26;27(1):108777. doi: 10.1016/j.isci.2023.108777. eCollection 2024 Jan 19. iScience. 2023. PMID: 38269097 Free PMC article.
-
Proteomic and phosphoproteomic analyses reveal that TORC1 is reactivated by pheromone signaling during sexual reproduction in fission yeast.PLoS Biol. 2024 Dec 20;22(12):e3002963. doi: 10.1371/journal.pbio.3002963. eCollection 2024 Dec. PLoS Biol. 2024. PMID: 39705284 Free PMC article.
-
The Catalytic Subunit of Schizosaccharomyces pombe CK2 (Cka1) Negatively Regulates RNA Polymerase II Transcription through Phosphorylation of Positive Cofactor 4 (PC4).Int J Mol Sci. 2022 Aug 22;23(16):9499. doi: 10.3390/ijms23169499. Int J Mol Sci. 2022. PMID: 36012759 Free PMC article.
-
A distinct phase of cyclin B (Cdc13) nuclear export at mitotic entry in S. pombe.bioRxiv [Preprint]. 2025 Aug 7:2025.06.05.658100. doi: 10.1101/2025.06.05.658100. bioRxiv. 2025. PMID: 40501888 Free PMC article. Preprint.
-
Novel TORC1 inhibitor Ecl1 is regulated by phosphorylation in fission yeast.Aging Cell. 2025 Apr;24(4):e14450. doi: 10.1111/acel.14450. Epub 2025 Feb 5. Aging Cell. 2025. PMID: 39910760 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

